Volume 9, Issue 4 (10-2023)
Journal of Research in Applied and Basic Medical Sciences 2023, 9(4): 200-209 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Arora Sehgal S, Malik G, Atri R, Kaur P, Malik R, Sehgal S, et al . Complaisance of COVID-19 vaccination in cancer patients: A North Indian Tertiary Cancer Center (TCC) annotation. Journal of Research in Applied and Basic Medical Sciences 2023; 9 (4) :200-209
URL: http://ijrabms.umsu.ac.ir/article-1-281-en.html
URL: http://ijrabms.umsu.ac.ir/article-1-281-en.html
Shailley Arora Sehgal * 

 , Garima Malik
, Garima Malik 

 , Rajeev Atri
, Rajeev Atri 

 , Paramjeet Kaur
, Paramjeet Kaur 

 , Rakesh Malik
, Rakesh Malik 

 , Sachin Sehgal
, Sachin Sehgal 

 , Vivek Kaushal
, Vivek Kaushal 

 , Ashok Chauhan
, Ashok Chauhan 




 , Garima Malik
, Garima Malik 

 , Rajeev Atri
, Rajeev Atri 

 , Paramjeet Kaur
, Paramjeet Kaur 

 , Rakesh Malik
, Rakesh Malik 

 , Sachin Sehgal
, Sachin Sehgal 

 , Vivek Kaushal
, Vivek Kaushal 

 , Ashok Chauhan
, Ashok Chauhan 


Assistant Professor, Department of Radiation Oncology, Regional Cancer Center, Pt B D Sharma PGIMS, Rohtak (Haryana), India , drshailley@gmail.com
Full-Text [PDF 689 kb]
(1048 Downloads)
| Abstract (HTML) (2077 Views)
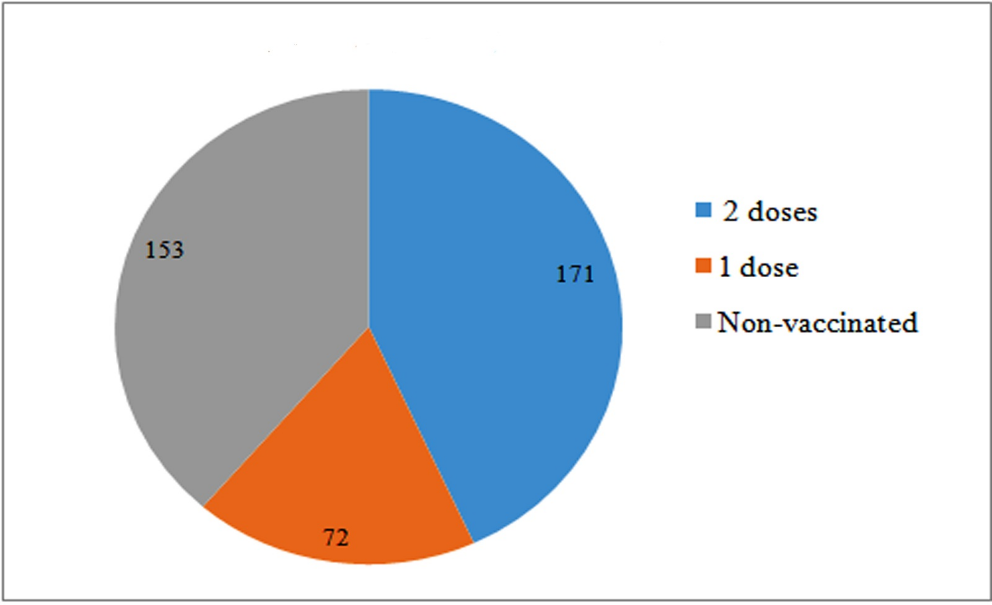



Fig. 4. District wise distribution of cancer patients with their vaccination status
Discussion
Even after prioritizing COVID-19 vaccination in cancer patients, the compliance and actual vaccination rates in Indian Cancer patients are a huge dilemma. Hence, this study was a continuum in research to assess acceptance of COVID-19 vaccination in a tertiary care cancer center of North India which caters mostly cancer patients hailing from rural background. The aim of this prospective observational study was to assess the compliance, coverage, and hesitancy of COVID-19 vaccination in cancer patients attending a tertiary care cancer center in North India. We interviewed 220 patients with confirmed histopathological diagnosis of malignancy who consented to participate in the study, and collected data on their demographic characteristics, type and site of malignancy, and vaccination status. We also analyzed the factors associated with vaccine acceptance or resistance among the cancer patients.
Mortality of cancer patients with COVID-19 infection is on the higher side compared to general population, as suggested by Wang et al. in his studies in which the case fatality rate of cancer patients with COVID-19 infection was 14.8% and 14.93%, while for noncancer patients it was around 4.1% and 5.26%, respectively in 2020 and 2021 studies (17, 18). Therefore, mitigating the risk of this infection for cancer patients is of utmost importance especially those receiving any sort of cancer therapy. The sole recommended way of tackling this pandemic is a robust vaccination campaign covering the entire population to achieve herd immunity. Efficacy of protection from COVID-19 infection was also documented in cancer patients. In a series of 1503 cancer patients from a cancer center in France, reduced SARS-CoV-2 infection and death were reported after two doses of COVID-19 vaccines, confirming that vaccines do work in actively treated cancer patients (19).
After starting COVID-19 vaccination drive in India on 16 January 2021, 200 crore vaccination doses were delivered to Indian citizens in 18 months i.e., till July 2022 but data in cancer patients especially in rural regions is scarce (20). India’s national COVID‑19 vaccination strategy hierarchized patients with high-risk conditions including patients with malignancy to be allocated for vaccination in phase II (4). Studies on vaccination of cancer patients showed much less coverage for example an Indian study done on 752 patients from January-June 2021 in a TCC showed 29.1%, and 7.6% of the patients received first and second doses of vaccine, respectively (21). Although vaccination drive was hastened up by Indian Government by using door-to-door campaign called Har Ghar Dastak in November 2021 to encourage people to get fully vaccinated and till December 2022, 69% of people in India have received at least one vaccine dose, and 64% are fully vaccinated as per data by Institute for Health Metrics and Evaluation, but vaccination coverage in cancer patients is still unknown (22). The COVID-19 Trends and Impact Survey in India has reported a vaccine acceptance rate of 77% among the Indian population and a vaccine hesitancy rate of 23% among respondents, where most of the hesitant people wanted to 'wait and see' (23).
Recent studies across the Globe have shown that vaccine acceptance rates are on an increasing trend in general population as shown in a global survey conducted by Lazarus et al. in 2022 with 23,000 respondents from 23 countries including India which demonstrated vaccine acceptance in general population has increased from 75.2% to 79.1% from 2020 to 2022 (24). But the compliance towards the vaccination is less in cancer patients as compared to the general population. Various studies reported different rates of vaccination among cancer patients around the world, depending on the availability of vaccines, the type and stage of cancer, the timing and modality of treatment, the socio-demographic characteristics, and the knowledge and attitude of the patients. In our study, we found that the vaccine acceptance rate among cancer patients was 61.36%, which is lower than the reported rates in the USA (83%) (25) but higher than the reported rates in few countries such as Tunisia (50.5%) (26) and France (53.7%) (27).
A big snag in the way of successful vaccination campaigns in cancer patients is lack of availability of vaccines and awareness at remote regions, fear of side effects, hesitancy regarding effectiveness of the vaccine and poor general condition of cancer patients due to cancer per se and/or related to treatment. An exploratory analysis by Dhalaria et al., on vaccine coverage and hesitancy in India revealed that COVID-19 vaccine hesitancy has a negative and statistically significant impact on COVID-19 vaccination coverage (28). In the present study, it was found that age is the only factor which is statistically significant, and maximum vaccination coverage was seen amongst ≥ 80 years age group i.e., 70%, although only 10 patients were there in this age group. This is congruent with the Government of India’s vaccine age-wise roll out strategy. Although age group, wise differences were statistically insignificant. We found gender vise differences in vaccination, males’ vaccination coverage being higher than females but it was statistically insignificant. This may be due to differences in education and awareness amongst females. Vaccine defiance factors reported in the present study are akin to previous such studies in the general population, such as age, gender, etc. (23, 26, 28).
In this pestilent era, almost every cancer patient has done some confabulation with their treating oncologist regarding the necessity of vaccination against COVID-19 infection. Hence this is the primary incumbency of the oncologist to step in and remove this vaccine skepticism by sensitizing their patients regarding the momentousness of vaccination in averting severe disease as well as mortality due to this deadly viral infection. in advocating for cancer patients to receive the available COVID-19 vaccines (25, 27, 29). Although most registered vaccine trials debar cancer patients, adding in the precariousness of oncologists to prescribe COVID-19 vaccine to their patients, but springing novel real-world data have provided ample evidence for solace. Centers for Disease Control and Prevention (CDC) have also endorsed vaccination for cancer patients, despite speculations regarding its safety (30).
Flaws of the present study also need to be stressed to have space for further amelioration. This study had limited sample size and the study population was mostly from the rural background, hence, the results can’t be generalized to the entire cancer patient population. Secondly, as it is a cross-sectional study, the scenario may only be stated at the time of the study. Lastly, the validated questionnaire is lacking to look for the cause of hesitancy in this set of population. Hence, more elaborate clinical trials entailing a large cancer patient population with a well-framed set of survey questions are advocated to throw more clarity in this field and remove vaccine hesitancy.
Conclusion
Devastating combination of cancer and COVID-19 infection is already a double-edged sword for cancer patients. Hence COVID-19 vaccine resistance in the cancer patients needs to be overcome with the proactive efforts put together by the treating oncologists, government, and the family of the cancer patients, to dwindle the effects of this fatal viral infection. This study accentuated the real picture and on-ground reality of COVID-19 vaccination acceptance among the Indian cancer patient population, thus making it feasible to figure out the necessary measures to hasten this vaccination campaign in such patients.
Acknowledgments
We sincerely thank all the collaborators who contributed to this research.
Conflict of interest
No conflict of interest declaration between the authors.
Funding/support
The Regional Cancer Center Haryana provided financial support for this study.
Full-Text: (809 Views)
Introduction
Since its emergence in December 2019, Coronavirus disease 2019 (COVID-19) pandemic has impacted around 608 million cases worldwide till 2023 February 25, creating major upheavals across the globe (1). As of February 2023, approximately 44 million confirmed COVID-19 cases have been reported in India (1). Standard preventive measures like social distancing, wearing N-95 masks, hand hygiene, etc., and vaccination are the only ways of thwarting the chances of getting COVID-19 infection. In 2020, approximately 19.3 million new cancer cases were reported globally as per GLOBOCAN, which posed a significant challenge to health care systems across the world to protect this vulnerable and immunocompromised population from COVID-19 infection (2). Regional Cancer Centre (RCC) of Rohtak city is one of the very few Government-run Tertiary Cancer Centers (TCC) in the state of Haryana, regaling roughly about 25 million people of Haryana State and neighborhood (3). Accretion of around 4,000 incident cancer cases per year mostly belonging to the proletarian strata for the treatment enrollment in this Cancer center, heap up the cancer load and makes it challenging for the oncologists to strike the balance between the perpetual cancer care and containment of the spread of COVID-19 infection among such immunocompromised patients.
Key strategy to combat COVID-19 infection started with the roll out of one of the biggest mass vaccination programs in the world around early December 2020 and in India it was started on 16 January 2021. The vaccines namely ChAdOx1 nCoV-19 (Covishield), BBV152 (Covaxin), Gam-COVID-Vac (Sputnik V), mRNA-1273 (Moderna), Ad26.COV2.S (Janssen), and DNA plasmid vaccine (ZyCoV-D ) were approved for emergency use authorization by Central Drugs Standard Control Organization (CDSCO) until August 2021, and in early 2022, emergency use approvals were granted to BECOV2D (Corbevax), SARS-CoV-2Rs recombinant spike protein Nanoparticle Vaccine (Covovax), and (Sputnik Light vaccines) (4).
Cancer patients are at high risk of severe COVID-19 complications like stroke, embolism, thrombosis, etc. (5). The death probability among cancer patients infected with COVID-19 infection was estimated to be 25.6% (6). Moreover, recent chemotherapy increases the risk of severe COVID-19 infection (7). As per the South Asian Declaration of the Consensus Guidelines for COVID-19 vaccination in cancer patients, the benefits of getting vaccination shots outweigh the risks, although discussion with the treating oncologist is warranted (8). Oncological societies across the world have emphasized the need of priority vaccination campaign for cancer patients especially hematologic malignancies, the ones with poor performance status and recently treated with chemotherapy (8-12). Despite all these strengthening facts and debates warranting the vaccination in cancer patients, certain logistics also play a crucial role e.g., vaccine trials encompassing mild or no cancer patients questioning their safety especially in this susceptible population. Also, there is scarce data on separate analysis for their efficacy or immune response assessment in such patients (12-15). Recently new vaccine trial data showing up to 8% cancer patients in their subgroup analysis, but they included stable disease at baseline who were not receiving immunosuppressive therapy (16). Thus, till today there is no convincing data to substantiate vaccine safety and efficacy in the patients with cancer.
Although vaccine acceptance and compliance studies have been reported worldwide, there is limited research in this area from the Indian subcontinent where majority of population is rural and lack of education, awareness, poor access to health care facilities, and digital illiteracy are major concerns (4). Hence the present research is an effort to study the vaccine coverage adequacy, hesitancy, and compliance of cancer patients towards COVID-19 vaccination. Adequate and prompt vaccine coverage is mandatory in cancer patients and vaccine-related amenability and related concerns need to be reported and well documented so that efforts can be taken to increase the admissibility of cancer patients. Thus, aim of conducting the present study was to showcase the acceptance of Indian cancer center patient population to COVID-19 vaccination.
Materials & Methods
The present prospective-observational research was done at TCC to appraise the compliance to COVID-19 vaccination in cancer patients. The study was conducted over a period of five months, i.e. from October 2021 to February 2022. Ethical clearance was taken from the Departmental Ethical Committee before commencement of the study. Patients with confirmed histopathological diagnosis of malignancy attending outpatient facility at TCC who consented were interviewed in person by universal sampling technique, regardless of type of vaccine which they received. Semi-structured interview was used to collect information. First part of this semi-structured interview format was related to demographics like the age, gender, rural/urban background, district and site of malignancy. Second part included information regarding Covid-19 vaccination asking date, time and place of vaccination including additional comments. The primary endpoint was the proportion of cancer patients attending the TCC who received single or both doses of COVID‑19 vaccine.
Statistical Analysis:
Data entered was analyzed using Statistical Package for Social Sciences (SPSS) version 25 for Windows. Quantitative data presented as mean and standard deviation (SD). Qualitative data presented as ratios and percentages. Qualitative data were compared using Chi-square test. The Chi-square test was used to determine correlation between the different demographic indices and the vaccination status. p value <0.05 was taken as the level of significance. The hypothesis used was no difference in vaccination based on particular demographic index e.g., gender.
Results
After scrutinizing the patients attending the outpatient facility from October 2021 to February 2022, only 396 patients consented for the interview for present study. 396 patients who participated in this study were of variable age groups starting from 16 to 92 years, with an overall mean age of 55.96 years and median age of 56 years. Demographic indices of the study population are tabulated in Table 1.
Since its emergence in December 2019, Coronavirus disease 2019 (COVID-19) pandemic has impacted around 608 million cases worldwide till 2023 February 25, creating major upheavals across the globe (1). As of February 2023, approximately 44 million confirmed COVID-19 cases have been reported in India (1). Standard preventive measures like social distancing, wearing N-95 masks, hand hygiene, etc., and vaccination are the only ways of thwarting the chances of getting COVID-19 infection. In 2020, approximately 19.3 million new cancer cases were reported globally as per GLOBOCAN, which posed a significant challenge to health care systems across the world to protect this vulnerable and immunocompromised population from COVID-19 infection (2). Regional Cancer Centre (RCC) of Rohtak city is one of the very few Government-run Tertiary Cancer Centers (TCC) in the state of Haryana, regaling roughly about 25 million people of Haryana State and neighborhood (3). Accretion of around 4,000 incident cancer cases per year mostly belonging to the proletarian strata for the treatment enrollment in this Cancer center, heap up the cancer load and makes it challenging for the oncologists to strike the balance between the perpetual cancer care and containment of the spread of COVID-19 infection among such immunocompromised patients.
Key strategy to combat COVID-19 infection started with the roll out of one of the biggest mass vaccination programs in the world around early December 2020 and in India it was started on 16 January 2021. The vaccines namely ChAdOx1 nCoV-19 (Covishield), BBV152 (Covaxin), Gam-COVID-Vac (Sputnik V), mRNA-1273 (Moderna), Ad26.COV2.S (Janssen), and DNA plasmid vaccine (ZyCoV-D ) were approved for emergency use authorization by Central Drugs Standard Control Organization (CDSCO) until August 2021, and in early 2022, emergency use approvals were granted to BECOV2D (Corbevax), SARS-CoV-2Rs recombinant spike protein Nanoparticle Vaccine (Covovax), and (Sputnik Light vaccines) (4).
Cancer patients are at high risk of severe COVID-19 complications like stroke, embolism, thrombosis, etc. (5). The death probability among cancer patients infected with COVID-19 infection was estimated to be 25.6% (6). Moreover, recent chemotherapy increases the risk of severe COVID-19 infection (7). As per the South Asian Declaration of the Consensus Guidelines for COVID-19 vaccination in cancer patients, the benefits of getting vaccination shots outweigh the risks, although discussion with the treating oncologist is warranted (8). Oncological societies across the world have emphasized the need of priority vaccination campaign for cancer patients especially hematologic malignancies, the ones with poor performance status and recently treated with chemotherapy (8-12). Despite all these strengthening facts and debates warranting the vaccination in cancer patients, certain logistics also play a crucial role e.g., vaccine trials encompassing mild or no cancer patients questioning their safety especially in this susceptible population. Also, there is scarce data on separate analysis for their efficacy or immune response assessment in such patients (12-15). Recently new vaccine trial data showing up to 8% cancer patients in their subgroup analysis, but they included stable disease at baseline who were not receiving immunosuppressive therapy (16). Thus, till today there is no convincing data to substantiate vaccine safety and efficacy in the patients with cancer.
Although vaccine acceptance and compliance studies have been reported worldwide, there is limited research in this area from the Indian subcontinent where majority of population is rural and lack of education, awareness, poor access to health care facilities, and digital illiteracy are major concerns (4). Hence the present research is an effort to study the vaccine coverage adequacy, hesitancy, and compliance of cancer patients towards COVID-19 vaccination. Adequate and prompt vaccine coverage is mandatory in cancer patients and vaccine-related amenability and related concerns need to be reported and well documented so that efforts can be taken to increase the admissibility of cancer patients. Thus, aim of conducting the present study was to showcase the acceptance of Indian cancer center patient population to COVID-19 vaccination.
Materials & Methods
The present prospective-observational research was done at TCC to appraise the compliance to COVID-19 vaccination in cancer patients. The study was conducted over a period of five months, i.e. from October 2021 to February 2022. Ethical clearance was taken from the Departmental Ethical Committee before commencement of the study. Patients with confirmed histopathological diagnosis of malignancy attending outpatient facility at TCC who consented were interviewed in person by universal sampling technique, regardless of type of vaccine which they received. Semi-structured interview was used to collect information. First part of this semi-structured interview format was related to demographics like the age, gender, rural/urban background, district and site of malignancy. Second part included information regarding Covid-19 vaccination asking date, time and place of vaccination including additional comments. The primary endpoint was the proportion of cancer patients attending the TCC who received single or both doses of COVID‑19 vaccine.
Statistical Analysis:
Data entered was analyzed using Statistical Package for Social Sciences (SPSS) version 25 for Windows. Quantitative data presented as mean and standard deviation (SD). Qualitative data presented as ratios and percentages. Qualitative data were compared using Chi-square test. The Chi-square test was used to determine correlation between the different demographic indices and the vaccination status. p value <0.05 was taken as the level of significance. The hypothesis used was no difference in vaccination based on particular demographic index e.g., gender.
Results
After scrutinizing the patients attending the outpatient facility from October 2021 to February 2022, only 396 patients consented for the interview for present study. 396 patients who participated in this study were of variable age groups starting from 16 to 92 years, with an overall mean age of 55.96 years and median age of 56 years. Demographic indices of the study population are tabulated in Table 1.
Table 1. Demographic indices of the study population
| Demographic Indices | No. of patients (396) |
| Male | 221(55.80%) |
| Female | 175(44.19%) |
| Rural | 292(73.74%) |
| Urban | 104(26.26%) |
| Age groups | |
| 16-24yrs | 5(1.26%) |
| 25-39yrs | 36(9.09%) |
| 40-49yrs | 66(16.67%) |
| 50-59yrs | 116(29.29%) |
| 60-69yrs | 113(28.53%) |
| 70-79yrs | 50(12.62%) |
| ≥80yrs | 10(2.52%) |
Patients with varied malignancy were interviewed however maximum patients were of head and neck malignancy i.e., 161 patients (40.66%) followed by breast malignancy i.e., 84 patients (21.21%) (Table 2).
Table 2. Site of Malignancy-wise distribution of study population
| S. No. | Type of Malignancy | No. of Patients | Percentage |
| 1 | Carcinoma Anal Canal | 5 | 1.26% |
| 2 | Carcinoma Breast | 84 | 21.21% |
| 3 | Brain Tumors | 2 | 0.51% |
| 4 | Gynecological Tumors | 16 | 4.04% |
| 5 | Carcinoma Colon | 2 | 0.51% |
| 6 | Carcinoma Esophagus | 23 | 5.81% |
| 7 | Carcinoma Endometrium | 2 | 0.51% |
| 8 | Carcinoma Gall Bladder | 8 | 2.02% |
| 9 | Carcinoma Gastroesophageal Junction | 1 | 0.25% |
| 10 | Head and Neck Cancers | 161 | 40.66% |
| 11 | Carcinoma Lung | 13 | 3.28% |
| 12 | Carcinoma Ovary | 21 | 5.30% |
| 13 | Carcinoma Pancreas | 2 | 0.51% |
| 14 | Carcinoma Penis | 1 | 0.25% |
| 15 | Carcinoma Prostate | 4 | 1.01% |
| 16 | Carcinoma Rectum | 4 | 1.01% |
| 17 | Carcinoma Stomach | 1 | 0.25% |
| 18 | Carcinoma Small Intestine | 1 | 0.25% |
| 19 | Testicular Tumor | 2 | 0.51% |
| 20 | Carcinoma Urinary Bladder | 9 | 2.27% |
| 21 | Soft Tissue Sarcoma | 8 | 2.02% |
| 22 | Hodgkins Lymphoma | 2 | 0.51% |
| 23 | Non-Hodgkins Lymphoma | 6 | 1.52% |
| 24 | Renal Cell Carcinoma | 4 | 1.01% |
| 25 | Osteosarcoma | 1 | 0.25% |
| 26 | Ewing’s Sarcoma | 3 | 0.76% |
| 27 | Metastasis of Unknown Origin | 12 | 3.03% |
In total, 243 i.e., 61.36% out of 396 patients were vaccinated either with single or both doses of COVID-19 vaccine, and 153 patients i.e., 38.64% were not vaccinated. Out of 243 vaccinated patients, 171 i.e., 70.37% patients were fully vaccinated with 2 doses and 72 patients i.e., 29.63% were vaccinated with single dose of COVID-19 vaccination irrespective of type of vaccine. Figure 1 shows the pie chart representation of the proportion of vaccinated and non-vaccinated cancer patients.

Fig. 1. Pie chart representation of vaccination status of cancer patients
Although the number of males vaccinated was higher than females but it was statistically insignificant (p = 0.62). Graphic portrayal of gender-wise distribution of study population among vaccinated versus non-vaccinated patients is given in Figure 2.

Fig. 2. Gender-wise distribution of patients included in the study (p = 0.62)
Mean age of the vaccinated patients was 57.16 ± 12.83 versus 54.14 ± 12.58 in non-vaccinated patients (p = 0.022). As the study population had variable age groups, their vaccination status was compared with this variability but didn’t show significant difference (p = 0.169) (Figure 3). Maximum patients were in 50-59 years age group i.e., 116 (29.29%). Amongst them only 39.66 % of the patients were fully vaccinated. Maximum vaccination coverage was seen amongst ≥ 80 years age group i.e., 90% but only 10 patients were there in this age group followed by 70-79-year age group with 70% patients being vaccinated with single or both doses.

Fig. 3. Age group wise distribution of cancer patients along with their vaccination status
Study population belonged to the rural background mostly amounting 73.74% (292) of total patients but only 178 (61%) were vaccinated. While 65 (62.5%) were vaccinated amongst 104 urban patients (p = 0.78).
Study population belonged to the rural background mostly amounting 73.74% (292) of total patients but only 178 (61%) were vaccinated. While 65 (62.5%) were vaccinated amongst 104 urban patients (p = 0.78).
District wise distribution was also analyzed to know the vaccine coverage status of various regions of Haryana and nearby states (Figure 4). Maximum patients hailed from Rohtak district followed by Sonipat and Jind. Out of total vaccinated cancer patients, maximum patients were also from Rohtak district i.e., 46 people (18.93%) (Figure 4).

Fig. 4. District wise distribution of cancer patients with their vaccination status
Discussion
Even after prioritizing COVID-19 vaccination in cancer patients, the compliance and actual vaccination rates in Indian Cancer patients are a huge dilemma. Hence, this study was a continuum in research to assess acceptance of COVID-19 vaccination in a tertiary care cancer center of North India which caters mostly cancer patients hailing from rural background. The aim of this prospective observational study was to assess the compliance, coverage, and hesitancy of COVID-19 vaccination in cancer patients attending a tertiary care cancer center in North India. We interviewed 220 patients with confirmed histopathological diagnosis of malignancy who consented to participate in the study, and collected data on their demographic characteristics, type and site of malignancy, and vaccination status. We also analyzed the factors associated with vaccine acceptance or resistance among the cancer patients.
Mortality of cancer patients with COVID-19 infection is on the higher side compared to general population, as suggested by Wang et al. in his studies in which the case fatality rate of cancer patients with COVID-19 infection was 14.8% and 14.93%, while for noncancer patients it was around 4.1% and 5.26%, respectively in 2020 and 2021 studies (17, 18). Therefore, mitigating the risk of this infection for cancer patients is of utmost importance especially those receiving any sort of cancer therapy. The sole recommended way of tackling this pandemic is a robust vaccination campaign covering the entire population to achieve herd immunity. Efficacy of protection from COVID-19 infection was also documented in cancer patients. In a series of 1503 cancer patients from a cancer center in France, reduced SARS-CoV-2 infection and death were reported after two doses of COVID-19 vaccines, confirming that vaccines do work in actively treated cancer patients (19).
After starting COVID-19 vaccination drive in India on 16 January 2021, 200 crore vaccination doses were delivered to Indian citizens in 18 months i.e., till July 2022 but data in cancer patients especially in rural regions is scarce (20). India’s national COVID‑19 vaccination strategy hierarchized patients with high-risk conditions including patients with malignancy to be allocated for vaccination in phase II (4). Studies on vaccination of cancer patients showed much less coverage for example an Indian study done on 752 patients from January-June 2021 in a TCC showed 29.1%, and 7.6% of the patients received first and second doses of vaccine, respectively (21). Although vaccination drive was hastened up by Indian Government by using door-to-door campaign called Har Ghar Dastak in November 2021 to encourage people to get fully vaccinated and till December 2022, 69% of people in India have received at least one vaccine dose, and 64% are fully vaccinated as per data by Institute for Health Metrics and Evaluation, but vaccination coverage in cancer patients is still unknown (22). The COVID-19 Trends and Impact Survey in India has reported a vaccine acceptance rate of 77% among the Indian population and a vaccine hesitancy rate of 23% among respondents, where most of the hesitant people wanted to 'wait and see' (23).
Recent studies across the Globe have shown that vaccine acceptance rates are on an increasing trend in general population as shown in a global survey conducted by Lazarus et al. in 2022 with 23,000 respondents from 23 countries including India which demonstrated vaccine acceptance in general population has increased from 75.2% to 79.1% from 2020 to 2022 (24). But the compliance towards the vaccination is less in cancer patients as compared to the general population. Various studies reported different rates of vaccination among cancer patients around the world, depending on the availability of vaccines, the type and stage of cancer, the timing and modality of treatment, the socio-demographic characteristics, and the knowledge and attitude of the patients. In our study, we found that the vaccine acceptance rate among cancer patients was 61.36%, which is lower than the reported rates in the USA (83%) (25) but higher than the reported rates in few countries such as Tunisia (50.5%) (26) and France (53.7%) (27).
A big snag in the way of successful vaccination campaigns in cancer patients is lack of availability of vaccines and awareness at remote regions, fear of side effects, hesitancy regarding effectiveness of the vaccine and poor general condition of cancer patients due to cancer per se and/or related to treatment. An exploratory analysis by Dhalaria et al., on vaccine coverage and hesitancy in India revealed that COVID-19 vaccine hesitancy has a negative and statistically significant impact on COVID-19 vaccination coverage (28). In the present study, it was found that age is the only factor which is statistically significant, and maximum vaccination coverage was seen amongst ≥ 80 years age group i.e., 70%, although only 10 patients were there in this age group. This is congruent with the Government of India’s vaccine age-wise roll out strategy. Although age group, wise differences were statistically insignificant. We found gender vise differences in vaccination, males’ vaccination coverage being higher than females but it was statistically insignificant. This may be due to differences in education and awareness amongst females. Vaccine defiance factors reported in the present study are akin to previous such studies in the general population, such as age, gender, etc. (23, 26, 28).
In this pestilent era, almost every cancer patient has done some confabulation with their treating oncologist regarding the necessity of vaccination against COVID-19 infection. Hence this is the primary incumbency of the oncologist to step in and remove this vaccine skepticism by sensitizing their patients regarding the momentousness of vaccination in averting severe disease as well as mortality due to this deadly viral infection. in advocating for cancer patients to receive the available COVID-19 vaccines (25, 27, 29). Although most registered vaccine trials debar cancer patients, adding in the precariousness of oncologists to prescribe COVID-19 vaccine to their patients, but springing novel real-world data have provided ample evidence for solace. Centers for Disease Control and Prevention (CDC) have also endorsed vaccination for cancer patients, despite speculations regarding its safety (30).
Flaws of the present study also need to be stressed to have space for further amelioration. This study had limited sample size and the study population was mostly from the rural background, hence, the results can’t be generalized to the entire cancer patient population. Secondly, as it is a cross-sectional study, the scenario may only be stated at the time of the study. Lastly, the validated questionnaire is lacking to look for the cause of hesitancy in this set of population. Hence, more elaborate clinical trials entailing a large cancer patient population with a well-framed set of survey questions are advocated to throw more clarity in this field and remove vaccine hesitancy.
Conclusion
Devastating combination of cancer and COVID-19 infection is already a double-edged sword for cancer patients. Hence COVID-19 vaccine resistance in the cancer patients needs to be overcome with the proactive efforts put together by the treating oncologists, government, and the family of the cancer patients, to dwindle the effects of this fatal viral infection. This study accentuated the real picture and on-ground reality of COVID-19 vaccination acceptance among the Indian cancer patient population, thus making it feasible to figure out the necessary measures to hasten this vaccination campaign in such patients.
Acknowledgments
We sincerely thank all the collaborators who contributed to this research.
Conflict of interest
No conflict of interest declaration between the authors.
Funding/support
The Regional Cancer Center Haryana provided financial support for this study.
Type of Study: orginal article |
Subject:
Radiotherapy
References
1. WHO Coronavirus (COVID-19) Dashboard [Internet]. Geneva: World Health Organization; 2020 [2023 february 25]. Available from: https://covid19.who.int/. [URL]
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49. [DOI:10.3322/caac.21660] [PMID]
3. Malik G, Sehgal SA, Dhull AK, Chauhan AK, Kaushal V. Preliminary Approach to Cancer Patient Care During Unprecedented COVID-19 Pandemic. Clin Infect Immun 2020;5(3):55-8. [DOI:10.14740/cii113]
4. Purohit N, Chugh Y, Bahuguna P, Prinja S. COVID-19 management: The vaccination drive in India. Health Policy Technol 2022;11(2):100636. [DOI:10.1016/j.hlpt.2022.100636] [PMID] []
5. Stefanile A, Cellerino M, Koudriavtseva T. Elevated risk of thrombotic manifestations of Sars-cov-2 infection in cancer patients: A literature review. EXCLI Journal 2022;21:906-920. [DOI:10.17179/excli2022-5073]
6. Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, et al. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur J Cancer 2020;139:43-50. [DOI:10.1016/j.ejca.2020.08.011] [PMID] []
7. Grivas P, Khaki AR, Wise-Draper TM, French B, Hennessy C, Hsu CY, et al. Association of clinical factors and recent anti-cancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol:32(6):787-800. [DOI:10.1016/j.annonc.2021.02.024] [PMID] []
8. Thirumalairaj R, Parikh PM, Agarwal A, Singh R, Krishnamurthy A, Desai SS, et al. South Asian Declaration-consensus guidelines for COVID-19 vaccination in cancer patients. South Asian J Cancer 2021;10(1):3-8. [DOI:10.1055/s-0041-1731909] [PMID] []
9. Garassino MC, Vyas M, De Vries EG, Kanesvaran R, Giuliani R, Peters S. The ESMO call to action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann Oncol 2021;32(5):579-81. [DOI:10.1016/j.annonc.2021.01.068] [PMID] []
10. NCCN COVID-19 Vaccination guide for people with cancer. [Internet]. USA: National Comprehensive Cancer Network;2022 [2023 February 25]. https://www.nccn.org/docs/ default-source/covid-19/ covid-vaccine-and-cancer-05.pdf?sfvrsn=45cc3047_16. [URL]
11. Ministry of Health and Family Welfare India. Information Regarding COVID-19 Vaccine 2021 [Internet]. New Delhi: MOHFW Government of India; 2022 [2022 July]. https://main.mohfw.gov.in/newshighlights-31 [URL]
12. Gundavda MK, Gundavda KK. Cancer or COVID-19? A review of recommendations for COVID-19 vaccination in cancer patients. Curr Treat Options Oncol 2021;22(10):95. [DOI:10.1007/s11864-021-00903-7] [PMID] []
13. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383(27):2603-15. [DOI:10.1056/NEJMoa2034577] [PMID] []
14. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384(5):403-16. [DOI:10.1056/NEJMoa2035389] [PMID] []
15. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Eng J Med 2021;384(23):2187-201.
https://doi.org/10.1056/NEJMoa2101544 [DOI:10.1056/nejmoa2101544] [PMID] []
16. Thomas SJ, Perez JL, Lockhart SP, Hariharan S, Kitchin N, Bailey R, et al. Efficacy and safety of the BNT162b2 mRNA COVID-19 vaccine in participants with a history of cancer: subgroup analysis of a global phase 3 randomized clinical trial. Vaccine 2022;40(10):1483-92. [DOI:10.1016/j.vaccine.2021.12.046] [PMID] []
17. Wang Q, Berger NA, Xu R. When hematologic malignancies meet COVID-19 in the United States: infections, death and disparities. Blood Rev 2020;47:100775. [DOI:10.1016/j.blre.2020.100775] [PMID] []
18. Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol 2021;7(2):220-7. [DOI:10.1001/jamaoncol.2020.6178] [PMID] []
19. Heudel P, Favier B, Assaad S, Zrounba P, Blay JY. Reduced SARS-CoV-2 infection and death after two doses of COVID-19 vaccines in a series of 1503 cancer patients. Ann Oncol 2021;32(11):1443-4. [DOI:10.1016/j.annonc.2021.07.012] [PMID] []
20. Ministry of Health and Family Welfare. India's COVID-19 cumulative vaccine coverage. [Internet]. Delhi: Press Information Bureau; 2022 [2022 August]. https://pib.gov.in/PressReleaseIframePage.aspx?PRID=1846658. [URL]
21. Batra U, Nathany S, Bansal N, Sharma M. COVID-19 vaccination status in Indian patients with cancer: An observational study. Cancer Res Stat Treat 2021;4(2):219-23. [DOI:10.4103/crst.crst_131_21]
22. Institute of Health Metrics and Evaluation. COVID-19 Results Briefing India [Internet]. India:IHME;2022 [2022 June]. https://www.healthdata.org/sites/default/files/files/163_briefing_India_4.pdf. [URL]
23. UNICEF. Building confidence in the COVID-19 vaccine in India [Internet]. New Delhi: UNICEF India; 2021 [2022 July 15]. [DOI:10.31899/pgy15.1065]
24. Lazarus JV, Wyka K, White TM, Picchio CA, Gostin LO, Larson HJ, et al. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat Med 2023;29(2):366-75. [DOI:10.1038/s41591-022-02185-4] [PMID]
25. Kelkar AH, Blake JA, Cherabuddi K, Cornett H, McKee BL, Cogle CR. Vaccine enthusiasm and hesitancy in Cancer patients and the impact of a webinar. Healthcare 2021;9(3):351. [DOI:10.3390/healthcare9030351] [PMID] []
26. Mejri N, Berrazega Y, Ouertani E, Rachdi H, Bohli M, Kochbati L, et al. Understanding COVID-19 vaccine hesitancy and resistance: another challenge in cancer patients. Support Care Cancer 2022;30(1):289-93. [DOI:10.1007/s00520-021-06419-y] [PMID] []
27. Barrière J, Gal J, Hoch B, Cassuto O, Laysalle A, Chamorey E, et al. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann Oncol 2021;32(5):673-4. [DOI:10.1016/j.annonc.2021.01.066] [PMID] []
28. Dhalaria P, Arora H, Singh AK, Mathur M, S AK. COVID-19 vaccine hesitancy and vaccination coverage in India: An exploratory analysis. Vaccines 2022;10(5):739. [DOI:10.3390/vaccines10050739] [PMID] []
29. Brodziak A, Sigorski D, Osmola M, Wilk M, Gawlik-Urban A, Kiszka J, et al. Attitudes of patients with cancer towards vaccinations-results of online survey with special focus on the vaccination against COVID-19. Vaccines 2021;9(5):411. [DOI:10.3390/vaccines9050411] [PMID] []
30. Mandal A, Singh P, Samaddar A, Singh D, Verma M, Rakesh A, et al. Vaccination of cancer patients against COVID-19: towards the end of a dilemma. Med Oncol 2021;38(8):92. [DOI:10.1007/s12032-021-01540-8] [PMID] []
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




 gmail.com
gmail.com