Volume 9, Issue 4 (10-2023)
Journal of Research in Applied and Basic Medical Sciences 2023, 9(4): 272-279 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Makaju M, Luitel P, Nepal R, Kunwar S, Shrestha R K, Akela G, et al . Pattern of Serum Liver Enzymes in the Patients with Type 2 Diabetes Mellitus. Journal of Research in Applied and Basic Medical Sciences 2023; 9 (4) :272-279
URL: http://ijrabms.umsu.ac.ir/article-1-265-en.html
URL: http://ijrabms.umsu.ac.ir/article-1-265-en.html
Manila Makaju 

 , Pritika Luitel
, Pritika Luitel 

 , Rupa Nepal
, Rupa Nepal 

 , Saroj Kunwar
, Saroj Kunwar 

 , Ram Krishna Shrestha
, Ram Krishna Shrestha 

 , Govinda Akela
, Govinda Akela 

 , Suresh Jaiswal
, Suresh Jaiswal 

 , Rajesh Kumar Thakur *
, Rajesh Kumar Thakur * 




 , Pritika Luitel
, Pritika Luitel 

 , Rupa Nepal
, Rupa Nepal 

 , Saroj Kunwar
, Saroj Kunwar 

 , Ram Krishna Shrestha
, Ram Krishna Shrestha 

 , Govinda Akela
, Govinda Akela 

 , Suresh Jaiswal
, Suresh Jaiswal 

 , Rajesh Kumar Thakur *
, Rajesh Kumar Thakur * 


Department of Biochemistry, Modern Technical College, Pokhara University, Lalitpur, Nepal , npraj01@gmail.com
Full-Text [PDF 351 kb]
(717 Downloads)
| Abstract (HTML) (2200 Views)
Table 1. Baseline Characteristics by Gender
Table 2: Correlation of Biochemical Parameters between Case and Control
Table 3: Baseline characteristics based on Glycemic Status
.png)
Fig. 1. Correlation between HbA1C and ALP in Type 2 diabetic patients
.png)
Fig. 2. Correlation between AST and HbA1C
.png)
Fig. 3. Correlation between ALT and HbA1C
.png)
Fig. 4. Correlation between Fasting sugar and ALP
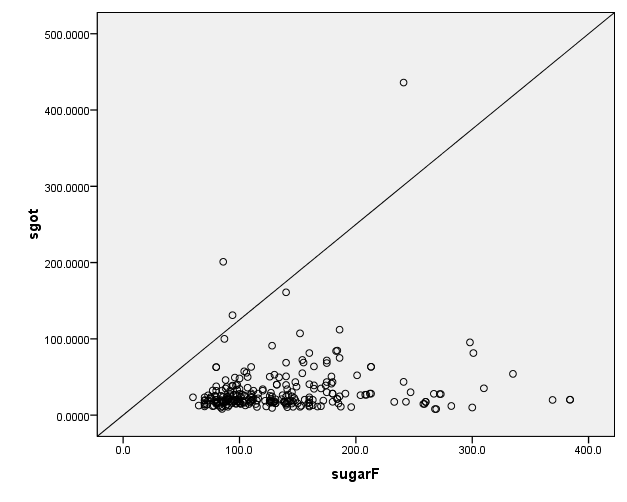
Fig. 5. Correlation between Fasting sugar and AST
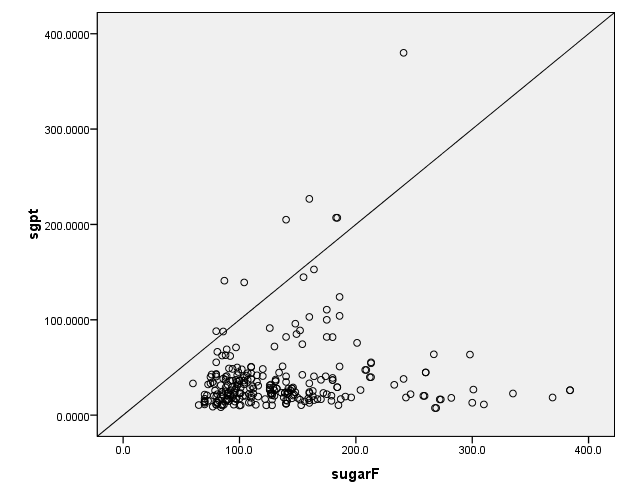
Fig. 6. Correlation between Fasting Sugar and ALT
Discussion
The prevalence of type 2 diabetes mellitus (T2DM) is around 4.5% in the adult population as per the data reported by IDF atlas 2013 in Nepal and its trend is escalating (14). A higher incidence of liver function test abnormalities has been associated with individuals suffering from T2DM than individuals without diabetes. In studies conducted by GetnetTeshome et al. and Shiful Islam et al. among diabetic patients, at least one liver enzyme was raised above the upper limit of the normal range (1, 15).
In our study, the prevalence of elevated liver enzymes is raised by ALT (28.8%), AST (23.7%), and ALP (48.8%) out of 135 Type II diabetic patients. Our results support the finding of a study done by Roshan Takelmayam et al. which also shows raised ALT and AST by 56% and 20%, respectively, although the study shows normal ALP levels (16). Similar study was conducted by Ghimire et al. in Nepal which reported a high prevalence of LFT abnormalities (about 62.3%) in 162 Type II diabetic patients, that have increased ALT, AST, and ALP by 15%, 13%, and 9% respectively (17). Also, Ni et al. performed a study on 81 type II diabetic patients in Malaysia, and reported that about 18%, 12%, and 15% have abnormal liver function tests in ALT, AST and ALP, respectively (18).
The increased incidence of LFT abnormalities has been associated with T2DM in studies conducted by Shiful Islam et al. in Bangladesh (1). These increase in liver enzymes in diabetic patients could be because of chronic disease and insulin resistance, leading to an increase of fatty acids with toxic effect on hepatocytes. All of these are responsible for the increase in transaminase and reduced synthetic functions of the liver (19).
The study conducted by Han Ni et al. in Myanmar shows raised levels of ALT and AST with 18.5 and 14.9 percents, respectively (18). Similarly, the same result was seen in the study by Sanjay Kumar Jha et al (20). Most of the previous studies analyzed AST, ALT, and GGT in T2D individuals, and only a few studies included ALP.
In contrast to our study, AST was not significantly correlated with HBA1C in a study conducted by AL-Jamil et al. in 2014 (21). A research from the western part of Nepal described a significant increase in ALT and ALP but AST did not elevate significantly in a diabetic patient when compared to the control group (22). A study conducted by Bora et al. in India and Balogun et al. in Nigeria reported a high prevalence of deranged LFTs of about 71.2% and 70%, respectively 23, 24).
The contrast in the above-mentioned study may be because of different populations, methodologies, and pathophysiological conditions.
Conclusion
In the present study, a significant increase in the level of liver enzymes, ALT, AST, and ALP was observed in type 2 diabetic patients when compared to healthy individuals. Hence, we found an association between the level of liver enzymes, ALT, AST, and ALP and type 2 diabetes mellitus.
Liver enzymes can be used as a biomarker for the assessment of type 2 diabetes, and it is possible to monitor complications of type 2 diabetes along with the study of serum liver enzymes through liver function tests.
Acknowledgments
The authors like to acknowledge all the helping hands that helped to shape up the work to make it successful.
Data availability
The raw data supporting the conclusions of this article are available from the authors upon reasonable request.
Conflict of interest
None of the authors have any interest that conflicts with this study.
Funding/support
Nine
Full-Text: (1076 Views)
Introduction
Diabetes mellitus (DM) is one of the major and common non-communicable diseases. Type 2 diabetes is the most common form, as is found in 90% of total cases in diabetic patients (1). Since the liver plays a major role in the regulation of carbohydrate metabolism, there exists an association between diabetes and liver injury (2). Type 2 diabetes is also characterized by hyperglycemia, and is linked with hyperlipidemia or the disturbance in various liver enzymes as shown in different studies. Disturbances in Liver function tests are recognized in some diabetic patients (3).
The liver enzymes, AST, ALT, and ALP are routinely measured to evaluate liver function. AST, ALT, and ALP are also considered the markers of the hepatocellular health (4). ALT is considered the most specific marker of the liver function test, as it is primarily found in the organ itself; however, AST and ALP are also found in other tissues and are so considered as the non-specific markers of the liver function tests (5). GGT is the least specific marker of the liver function test (LFT) in the context of this study, since various studies have shown its independent elevation without any correlation with type 2 diabetes. GGT is seen to be mainly elevated in the patients with pre-hepatic, hepatic, or post-hepatic illness (for example jaundice) (6).
LFTs includes measuring AST, ALT, ALP, and GGT. The type 2 diabetic patients more frequently had elevated ALT and GGT levels than those with type 1 diabetes (7). However, increases in LFTs were rarely more than twice the upper limit of normal (8). The management of liver injury associated with diabetes is a global problem until now, and successful diagnosis and treatment are not yet available (9).
Liver function tests can be performed in diabetic patients, and the prevalence can be measured. Increased activity of the liver enzymes such as AST, ALT, ALP, and γ-GT is the indicator of hepatocellular injury (10, 11). Increased activity of these markers are associated with insulin resistance, metabolic syndrome, and type 2 diabetes (12). The fasting glucose level of the patient with type 2 diabetes will be correlated with various liver enzymes such as AST, ALT, and ALP. GGT is also a marker of liver injury but, however, will be excluded in this study since it is the most non-specific marker of hepatocellular injury and is mostly related to the biliary tract function. Various studies have also known the independency of increase of GGT with type 2 diabetic patients (13).
The aim of this study was to correlate liver enzymes with type 2 diabetic patients attending Star Hospital. The correlation study of liver enzymes is found to be conducted previously worldwide but is not sufficient in the context of Nepal. The site of this study is chosen to be Star Hospital since there is sufficient patient flow with type 2 diabetes in this hospital which will be enough to conduct the study. Also, diabetic patients from different districts of Nepal are getting treatment from Star hospital, which will make it relevant to conduct the study among the patients from different places in Nepal.
Materials & Methods
A quantitative type of hospital-based cross-sectional study was carried out in the Department of Biochemistry, Modern Technical College, Sanepa, Lalitpur, Nepal, on Type 2 diabetic patients attending Star Hospital, Sanepa from June to August 2021. 260 participants were enrolled. Out of 135 typed 2 diabetic patients, 125 were control subjects who were healthy controls enrolled in the study by a convenient Sampling Technique whose age group was above 40 years.
Ethical consideration was obtained from the Institutional Review Committee (IRC) of Star Hospital Research Center (SHRC). Written consent was taken from Star Hospital. Oral and written consent were also obtained from the participants who were included in the study.
Venous blood was collected from the median cubital vein by sterilizing the local area with an alcohol pad. Then, the venous blood was allowed to rest for clot formation and serum were recovered by centrifugation at 2000 rpm for 5-10 minutes. Then the different parametric test was performed.
Blood glucose estimation was done by GOD/POD (glucose oxidase/peroxidase) method where GOD acts on glucose to form gluconic acid and hydrogen peroxide. Peroxidase acts as hydrogen peroxide to form water and oxygen. 4-amino antipyrine reacts with oxygen to form a pink colored complex. The formation of pink color is directly proportional to the concentration of glucose present in serum. The intensity of the color is measured at 540 nm of colorimeter.
Serum Alanine aminotransferase (ALT) estimation by IFCC method: ALT or SGPT catalyzes the transfer of amino group from alanine to α- ketoglutarate forming pyruvate and glutamate. The absorbance of the colored product is measured in a spectrophotometer at 340 nm wavelengths.
Serum Aspartate aminotransferase (AST) estimation by IFCC method: AST or SGOT catalyzes the transfer of the amino group from aspartate to α-ketoglutarate, forming oxaloacetate and glutamate. The absorbance of the color produced is measured in a spectrophotometer at 340 nm wavelength.
Serum Alkaline Phosphatase (ALP) estimation: Alkaline Phosphatase cleaves p-nitrophenyl phosphate (p-NPP) into p-nitrophenol and phosphate. P-nitrophenol is a yellow color compound in an alkaline medium and absorbs light at 405 nm. The rate of increase in absorbance at 405 nm is proportional to alkaline phosphatase in the specimen.
All the data were entered in the MS excel and further statistical analysis was done in the statistical package for social science (SPSS) version 16. Frequency and chi-square test were measured. P values less than 0.05 were considered significant.
Results
A total study comprises 260 participants; of which, 151 were male with mean age of 57.1±16.0 years and 109 were female with mean age of 58.2±16.2 years. ALT and AST of males are significantly higher than females, but changes in ALP level was insignificant as shown in table 1. Out of 135 cases of Type 2 diabetes, 74 were male and 61 were female with mean age of 60.3±15.1 years. Similarly, 125 controls were taken out, of which, 77 were male and 48 were female with mean age of 54.7±16.6. Significant elevation of AST and ALP was found in the case group compared to control group, and ALT was found insignificant, as shown in table 2. Significant elevation of AST, ALT, and ALP was found in the patients with poor glycemic control than the patients with good glycemic control, as shown in table 3.
A statistically significant correlation was found between HbA1C and ALP (r=0.141, p=0.023), as shown in figure 1. Similarly, positive Correlation was found between HbA1C and AST, which was statistically significant (r=0.161, p=0.010), as shown in figure 2. Positive statistically significant correlation was found between HbA1C and ALT, which was r=0.119 and p=0.016, as shown in figure 3. Positive Correlation was found between fasting sugar and ALP, which was statistically significant (r=0.162, p=0.009), as shown in figure 4. Positive Correlation was found between fasting sugar and AST, which was statistically significant (r=0.171, p=0.006), as shown in figure 5. Positive Correlation was found between fasting sugar and ALT, which was statistically significant (r=0.133, p=0.032), as shown in figure 6.
Diabetes mellitus (DM) is one of the major and common non-communicable diseases. Type 2 diabetes is the most common form, as is found in 90% of total cases in diabetic patients (1). Since the liver plays a major role in the regulation of carbohydrate metabolism, there exists an association between diabetes and liver injury (2). Type 2 diabetes is also characterized by hyperglycemia, and is linked with hyperlipidemia or the disturbance in various liver enzymes as shown in different studies. Disturbances in Liver function tests are recognized in some diabetic patients (3).
The liver enzymes, AST, ALT, and ALP are routinely measured to evaluate liver function. AST, ALT, and ALP are also considered the markers of the hepatocellular health (4). ALT is considered the most specific marker of the liver function test, as it is primarily found in the organ itself; however, AST and ALP are also found in other tissues and are so considered as the non-specific markers of the liver function tests (5). GGT is the least specific marker of the liver function test (LFT) in the context of this study, since various studies have shown its independent elevation without any correlation with type 2 diabetes. GGT is seen to be mainly elevated in the patients with pre-hepatic, hepatic, or post-hepatic illness (for example jaundice) (6).
LFTs includes measuring AST, ALT, ALP, and GGT. The type 2 diabetic patients more frequently had elevated ALT and GGT levels than those with type 1 diabetes (7). However, increases in LFTs were rarely more than twice the upper limit of normal (8). The management of liver injury associated with diabetes is a global problem until now, and successful diagnosis and treatment are not yet available (9).
Liver function tests can be performed in diabetic patients, and the prevalence can be measured. Increased activity of the liver enzymes such as AST, ALT, ALP, and γ-GT is the indicator of hepatocellular injury (10, 11). Increased activity of these markers are associated with insulin resistance, metabolic syndrome, and type 2 diabetes (12). The fasting glucose level of the patient with type 2 diabetes will be correlated with various liver enzymes such as AST, ALT, and ALP. GGT is also a marker of liver injury but, however, will be excluded in this study since it is the most non-specific marker of hepatocellular injury and is mostly related to the biliary tract function. Various studies have also known the independency of increase of GGT with type 2 diabetic patients (13).
The aim of this study was to correlate liver enzymes with type 2 diabetic patients attending Star Hospital. The correlation study of liver enzymes is found to be conducted previously worldwide but is not sufficient in the context of Nepal. The site of this study is chosen to be Star Hospital since there is sufficient patient flow with type 2 diabetes in this hospital which will be enough to conduct the study. Also, diabetic patients from different districts of Nepal are getting treatment from Star hospital, which will make it relevant to conduct the study among the patients from different places in Nepal.
Materials & Methods
A quantitative type of hospital-based cross-sectional study was carried out in the Department of Biochemistry, Modern Technical College, Sanepa, Lalitpur, Nepal, on Type 2 diabetic patients attending Star Hospital, Sanepa from June to August 2021. 260 participants were enrolled. Out of 135 typed 2 diabetic patients, 125 were control subjects who were healthy controls enrolled in the study by a convenient Sampling Technique whose age group was above 40 years.
Ethical consideration was obtained from the Institutional Review Committee (IRC) of Star Hospital Research Center (SHRC). Written consent was taken from Star Hospital. Oral and written consent were also obtained from the participants who were included in the study.
Venous blood was collected from the median cubital vein by sterilizing the local area with an alcohol pad. Then, the venous blood was allowed to rest for clot formation and serum were recovered by centrifugation at 2000 rpm for 5-10 minutes. Then the different parametric test was performed.
Blood glucose estimation was done by GOD/POD (glucose oxidase/peroxidase) method where GOD acts on glucose to form gluconic acid and hydrogen peroxide. Peroxidase acts as hydrogen peroxide to form water and oxygen. 4-amino antipyrine reacts with oxygen to form a pink colored complex. The formation of pink color is directly proportional to the concentration of glucose present in serum. The intensity of the color is measured at 540 nm of colorimeter.
Serum Alanine aminotransferase (ALT) estimation by IFCC method: ALT or SGPT catalyzes the transfer of amino group from alanine to α- ketoglutarate forming pyruvate and glutamate. The absorbance of the colored product is measured in a spectrophotometer at 340 nm wavelengths.
Serum Aspartate aminotransferase (AST) estimation by IFCC method: AST or SGOT catalyzes the transfer of the amino group from aspartate to α-ketoglutarate, forming oxaloacetate and glutamate. The absorbance of the color produced is measured in a spectrophotometer at 340 nm wavelength.
Serum Alkaline Phosphatase (ALP) estimation: Alkaline Phosphatase cleaves p-nitrophenyl phosphate (p-NPP) into p-nitrophenol and phosphate. P-nitrophenol is a yellow color compound in an alkaline medium and absorbs light at 405 nm. The rate of increase in absorbance at 405 nm is proportional to alkaline phosphatase in the specimen.
All the data were entered in the MS excel and further statistical analysis was done in the statistical package for social science (SPSS) version 16. Frequency and chi-square test were measured. P values less than 0.05 were considered significant.
Results
A total study comprises 260 participants; of which, 151 were male with mean age of 57.1±16.0 years and 109 were female with mean age of 58.2±16.2 years. ALT and AST of males are significantly higher than females, but changes in ALP level was insignificant as shown in table 1. Out of 135 cases of Type 2 diabetes, 74 were male and 61 were female with mean age of 60.3±15.1 years. Similarly, 125 controls were taken out, of which, 77 were male and 48 were female with mean age of 54.7±16.6. Significant elevation of AST and ALP was found in the case group compared to control group, and ALT was found insignificant, as shown in table 2. Significant elevation of AST, ALT, and ALP was found in the patients with poor glycemic control than the patients with good glycemic control, as shown in table 3.
A statistically significant correlation was found between HbA1C and ALP (r=0.141, p=0.023), as shown in figure 1. Similarly, positive Correlation was found between HbA1C and AST, which was statistically significant (r=0.161, p=0.010), as shown in figure 2. Positive statistically significant correlation was found between HbA1C and ALT, which was r=0.119 and p=0.016, as shown in figure 3. Positive Correlation was found between fasting sugar and ALP, which was statistically significant (r=0.162, p=0.009), as shown in figure 4. Positive Correlation was found between fasting sugar and AST, which was statistically significant (r=0.171, p=0.006), as shown in figure 5. Positive Correlation was found between fasting sugar and ALT, which was statistically significant (r=0.133, p=0.032), as shown in figure 6.
Table 1. Baseline Characteristics by Gender
| Parameters | Male | Female | P-value |
| Age(years) | 57.1±16.0 | 58.2±16.2 | 0.606 |
| Sugar F(mg/dl) | 140.1±65.6 | 130.1±51.5 | 0.188 |
| Sugar PP(mg/dl) | 193.2±107.8 | 184.4±93 | 0.493 |
| Hba1c(%) | 6.5±2.2 | 6.2±1.7 | 0.202 |
| AST(IU/L) | 23.9(18.0,35.6) | 19.0(15.5,28.3) | 0.006 |
| ALT(IU/L) | 29.3(20.2,42.4) | 23.0(16.3,35.9) | 0.009 |
| ALP(IU/L) | 222.7±80.7 | 247.3±180.4 | 0.140 |
Table 2: Correlation of Biochemical Parameters between Case and Control
| Parameters | Case | Control | P-value |
| Age(years) | 60.3±15.1 | 54.7±16.6 | 0.005 |
| Sugar F(mg/dl) | 176.7±57.8 | 92.2±14.6 | 0.000 |
| Sugar PP(mg/dl) | 264.6±89.3 | 108.9±19.2 | 0.000 |
| Hba1c(%) | 7.8±1.9 | 4.9±0.5 | 0.000 |
| AST(IU/L) | 24.2(17.2,40.1) | 20.0(16.1,27.5) | 0.04 |
| ALT(IU/L) | 26.5(19.8,44.0) | 26.0(16.3,40.0) | 0.113 |
| ALP(IU/L) | 260.9±170.5 | 203.4±61.6 | 0.000 |
Table 3: Baseline characteristics based on Glycemic Status
| Parameters | HbA1c>7.0% (Poor Glycemic Control) |
HbA1c<7% Good Glycemic Control) |
P-value |
| Age(years) | 60.3±15.5 | 54.7±16.6 | 0.005 |
| Sugar F(mg/dl) | 176.6±57.8 | 92.2±14.5 | 0.000 |
| Sugar PP(mg/dl) | 264.5±89.3 | 108.8±19.2 | 0.000 |
| HbA1c(%) | 7.8±1.9 | 4.9±0.6 | 0.000 |
| AST(IU/L) | 28.2(17.4,50.7) | 20.0(16.6,28.1) | 0.010 |
| ALT(IU/L) | 29.3(19.9,51.0) | 25.8(17.7,37.8) | 0.033 |
| ALP(IU/L) | 260.9±170.5 | 203.4±61.6 | 0.000 |
.png)
Fig. 1. Correlation between HbA1C and ALP in Type 2 diabetic patients
.png)
Fig. 2. Correlation between AST and HbA1C
.png)
Fig. 3. Correlation between ALT and HbA1C
.png)
Fig. 4. Correlation between Fasting sugar and ALP

Fig. 5. Correlation between Fasting sugar and AST

Fig. 6. Correlation between Fasting Sugar and ALT
Discussion
The prevalence of type 2 diabetes mellitus (T2DM) is around 4.5% in the adult population as per the data reported by IDF atlas 2013 in Nepal and its trend is escalating (14). A higher incidence of liver function test abnormalities has been associated with individuals suffering from T2DM than individuals without diabetes. In studies conducted by GetnetTeshome et al. and Shiful Islam et al. among diabetic patients, at least one liver enzyme was raised above the upper limit of the normal range (1, 15).
In our study, the prevalence of elevated liver enzymes is raised by ALT (28.8%), AST (23.7%), and ALP (48.8%) out of 135 Type II diabetic patients. Our results support the finding of a study done by Roshan Takelmayam et al. which also shows raised ALT and AST by 56% and 20%, respectively, although the study shows normal ALP levels (16). Similar study was conducted by Ghimire et al. in Nepal which reported a high prevalence of LFT abnormalities (about 62.3%) in 162 Type II diabetic patients, that have increased ALT, AST, and ALP by 15%, 13%, and 9% respectively (17). Also, Ni et al. performed a study on 81 type II diabetic patients in Malaysia, and reported that about 18%, 12%, and 15% have abnormal liver function tests in ALT, AST and ALP, respectively (18).
The increased incidence of LFT abnormalities has been associated with T2DM in studies conducted by Shiful Islam et al. in Bangladesh (1). These increase in liver enzymes in diabetic patients could be because of chronic disease and insulin resistance, leading to an increase of fatty acids with toxic effect on hepatocytes. All of these are responsible for the increase in transaminase and reduced synthetic functions of the liver (19).
The study conducted by Han Ni et al. in Myanmar shows raised levels of ALT and AST with 18.5 and 14.9 percents, respectively (18). Similarly, the same result was seen in the study by Sanjay Kumar Jha et al (20). Most of the previous studies analyzed AST, ALT, and GGT in T2D individuals, and only a few studies included ALP.
In contrast to our study, AST was not significantly correlated with HBA1C in a study conducted by AL-Jamil et al. in 2014 (21). A research from the western part of Nepal described a significant increase in ALT and ALP but AST did not elevate significantly in a diabetic patient when compared to the control group (22). A study conducted by Bora et al. in India and Balogun et al. in Nigeria reported a high prevalence of deranged LFTs of about 71.2% and 70%, respectively 23, 24).
The contrast in the above-mentioned study may be because of different populations, methodologies, and pathophysiological conditions.
Conclusion
In the present study, a significant increase in the level of liver enzymes, ALT, AST, and ALP was observed in type 2 diabetic patients when compared to healthy individuals. Hence, we found an association between the level of liver enzymes, ALT, AST, and ALP and type 2 diabetes mellitus.
Liver enzymes can be used as a biomarker for the assessment of type 2 diabetes, and it is possible to monitor complications of type 2 diabetes along with the study of serum liver enzymes through liver function tests.
Acknowledgments
The authors like to acknowledge all the helping hands that helped to shape up the work to make it successful.
Data availability
The raw data supporting the conclusions of this article are available from the authors upon reasonable request.
Conflict of interest
None of the authors have any interest that conflicts with this study.
Funding/support
Nine
Type of Study: orginal article |
Subject:
Other
References
1. Rahman S, Islam S, Haque T, Kathak RR, Ali N. Association between serum liver enzymes and hypertension: a cross-sectional study in Bangladeshi adults. BMC Cardiovasc Disord 2020;20:1-7. [DOI:10.1186/s12872-020-01411-6] [PMID] []
2. Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 1997;14(S5):S7-85. https://doi.org/10.1002/(SICI)1096-9136(199712)14:5+3.0.CO;2-R
https://doi.org/10.1002/(SICI)1096-9136(199712)14:5+3.0.CO;2-R [DOI:10.1002/(SICI)1096-9136(199712)14:5+3.0.CO;2-R]
3. Leevy CM, Ryan CM, Fineberg JC. Diabetes mellitus and liver dysfunction; etiologic and therapeutic considerations. Am J Med 1950;8(3):290-9. [DOI:10.1016/0002-9343(50)90062-3] [PMID]
4. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002;23(2):201-29. http://dx.doi.org/10.1210/edrv.23.2.0461. [DOI:10.1210/edrv.23.2.0461] [PMID]
5. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14(2):88-98. http://dx.doi.org/10.1038/nrendo.2017.151. [DOI:10.1038/nrendo.2017.151] [PMID]
6. Hanley AJG, Williams K, Festa A, Wagenknecht LE, D'Agostino RB Jr, Kempf J, et al. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 2004;53(10):2623-32. [DOI:10.2337/diabetes.53.10.2623] [PMID]
7. Kim DJ, Noh JH, Cho NH, Lee BW, Choi YH, Jung JH, et al. Serum gamma-glutamyltransferase within its normal concentration range is related to the presence of diabetes and cardiovascular risk factors. Diabet Med 2005;22(9):1134-40. [DOI:10.1111/j.1464-5491.2005.01581.x] [PMID]
8. Harris EH. Elevated liver function tests in type 2 diabetes. Clin Diabetes 2005;23(3):115-9. [DOI:10.2337/diaclin.23.3.115]
9. Seifu Daniel ZB. Impairment of liver function tests and lipid profiles in type 2 diabetic patients treated at the diabetic center in Tikur Anbessa specialized teaching hospital (tasth), Addis Ababa, Ethiopia. J Diabetes Metab 2014;5(11). [DOI:10.4172/2155-6156.1000454]
10. Erbey JR, Silberman C, Lydick E. Prevalence of abnormal serum alanine aminotransferase levels in obese patients and patients with type 2 diabetes. Am J Med 2000;109(7):588-90. [DOI:10.1016/S0002-9343(00)00602-1] [PMID]
11. Meybodi MA, Afkhami-Ardekani M, Rashidi M. Prevalence of abnormal serum alanine aminotransferase levels in type 2 diabetic patients in Iran. Pak J Biol Sci 2008;11(18):2274-7. [DOI:10.3923/pjbs.2008.2274.2277] [PMID]
12. Grove J, Daly AK, Bassendine MF, Day CP. Association of a tumor necrosis factor promoter polymorphism with susceptibility to alcoholic steatohepatitis. Hepatology 1997;26(1):143-6. [DOI:10.1002/hep.510260119] [PMID]
13. Stranges S, Trevisan M, Dorn JM, Dmochowski J, Donahue RP. Body fat distribution, liver enzymes, and risk of hypertension: evidence from the Western New York Study: Evidence from the western New York study. Hypertension 2005;46(5):1186-93. [DOI:10.1161/01.HYP.0000185688.81320.4d] [PMID] []
14. Gyawali B, Ferrario A, van Teijlingen E, Kallestrup P. Challenges in diabetes mellitus type 2 management in Nepal: a literature review. Glob Health Action 2016;9(1):31704. [DOI:10.3402/gha.v9.31704] [PMID] []
15. Teshome G, Ambachew S, Fasil A, Abebe M. Prevalence of liver function test abnormality and associated factors in Type 2 Diabetes Mellitus: A comparative cross-sectional study. Electron. J Int Fed Clin Chem Lab Med 2019;30(3):303-16. [PMID]
16. Takhelmayum R, Thanpari C, Singh TP. Liver dysfunction in diabetic patients admitted in referral hospital. Bali Med J 2014;3(3):122-4. [DOI:10.15562/bmj.v3i3.87]
17. Ghimire S, Shakya S, Shakya J, Acharya P, Pardhe BD. Abnormal liver parameters among individuals with type 2 diabetes mellitus Nepalese population. Biochem Pharmacol (Los Angel) 2018;7(1):2167-501. [DOI:10.4172/2167-0501.1000243]
18. Ni H, Soe HH, Htet A. Determinants of abnormal liver function tests in diabetes patients in Myanmar. Int J Diabetes Res 2012;1(3):36-41. [DOI:10.5923/j.diabetes.20120103.02]
19. Chatila R, West AB. Hepatomegaly and abnormal liver tests due to glycogenosis in adults with diabetes. Medicine 1996;75(6):327-33. [DOI:10.1097/00005792-199611000-00003] [PMID]
20. Jha SK, Yadav NK, Rizal S. Prevalence of elevated liver enzymes and its association with type 2 diabetes: A Descriptive Cross-Sectional Study among Nepalese Adults from Biratnagar, Nepal. Asian J Med Sci 2021;12(6):50-5. [DOI:10.3126/ajms.v12i6.37074]
21. Al-Jameil N, Khan FA, Arjumand S, Khan MF, Tabassum H. Dyslipidemia and its correlation with type 2 diabetic patients at different stages of proteinuria. Biomed Res 2014;25(3):327-31. [Google Scholar]
22. Thanpari C, Yadav N, Takhelmayum R, Shrewastwa M, Thapa P. Status of antioxidant and liver function in type-2 diabetic patients attending Nepalgunj Medical College. Bali Med J 2013;2:1-4. [URL]
23. Bora K, Borah M, Chutia H, Nath CK, Das D, Ruram AA. Presence of concurrent derangements of liver function tests in type 2 diabetes and their relationship with glycemic status: A retrospective observational study from Meghalaya. J Lab Physicians 2016;8(01):030-5. [DOI:10.4103/0974-2727.176227] [PMID] []
24. Balogun WO, Adeleye JO, Akinlade KS, Adedapo KS, Kuti M. Frequent occurrence of high gamma-glutamyl transferase and alanine amino transferase among Nigerian patients with type 2 diabetes. Afr J Med Med Sci 2008;37(2):177-83. [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




 gmail.com
gmail.com