Volume 10, Issue 2 (4-2024)
Journal of Research in Applied and Basic Medical Sciences 2024, 10(2): 205-212 |
Back to browse issues page
Ethics code: MC-421 (GMCS-2020)
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Lone P A, Khan M A, Malik Y P. Adverse effects of consumption of anabolic steroids on heart: An Experimental Study. Journal of Research in Applied and Basic Medical Sciences 2024; 10 (2) :205-212
URL: http://ijrabms.umsu.ac.ir/article-1-330-en.html
URL: http://ijrabms.umsu.ac.ir/article-1-330-en.html
Senior Resident, Department of Anatomy, Government Medical College, Anantnag , parvaizgull01@gmail.com
Full-Text [PDF 595 kb]
(906 Downloads)
| Abstract (HTML) (1319 Views)
In the present study, no apparent gross abnormality was noted. Light microscopy of cardiac tissue revealed normal histology of the heart in Group A (Control). Group B (ND) at the end of the 8thweek, shows intermuscular hemorrhages, congestion of myocardial vessels, separated cardiacmyocytes and fragmented muscle fibers, vesicular nuclei and severe degenerative changes.
Ren (22) showed that steroids alone can induce cardiomyocyte hypertrophy a dose much higher than that used in our study (15%) increase with nandrolone alone. The divergence may be the result of different study design (e.g., their study was performed in cell culture conditions); therefore, the doses of the hormones may not be comparable.
Abdelhafez (23) showed highly degenerated muscle fibers with areas of hemorrhage and widened endomysium. Also, she demonstrated a numerous pyknotic and karyolytic nuclei.
The same findings were obtained by Soliman (24) who found that nandrolone administration causes a 10-fold increase in heart collagen.
Parssinen (21) added that this effect tended to be dose-dependent. These short-term changes in collagen metabolism may be explained by increased anabolic effects in muscle. The same results were obtained by Soliman (24).
Also, Elgendy (25) reported hypertrophy and degeneration of both cardiac and skeletal muscles and explained this by its effect on the androgen receptors that are widely distributed in different types of muscles.
Ren (22) showed that steroids alone can induce cardiomyocyte hypertrophy a dose much higher than that used in our study (15%) increase with nandrolone alone. The divergence may be the result of different study design (e.g., their study was performed in cell culture conditions); therefore, the doses of the hormones may not be comparable.
Conclusion
According to this study, using nandrolone can cause cardiac abnormalities like severe degenerative changes, myocardial vascular congestion, and intermuscular hemorrhages. These findings are consistent with earlier research, which has suggested increased collagen and muscle fiber degeneration. As the main limitation of the current study was low study population, the study's findings highlight and recommended the significance of future research into the long-term effects of these medications and effects of toxic doses of nandrolone, as well as careful consideration of study design and dose.
Acknowledgments
We would like to thank the Faculty of the Postgraduate Department of Anatomy, Government Medical College Srinagar, India and all the scholars whose articles are cited and included in references.
Ethical statement
This study was conducted after approval by the ethics committee of Government Medical College, Srinagar with code of ethics MC-421 (GMCS-2020).
Data availability
The raw data supporting the conclusions of this article are available from the authors upon reasonable request.
Author contributions
All the authors contributed equally from study design, methodology, procurement of drug used, processing of tissues, results, discussion to final submission.
Funding/Support
None.
Conflict of interest
The authors have no conflict of interest in this study.
Full-Text: (823 Views)
Introduction
Nandrolone deconates are a group of synthetic derivative of testosterone that are used to maximize anabolic effects and minimize androgenic ones are known as anabolic androgenic steroids (1). Nandrolone decanoate has a half-life of six days, and is gradually released into the bloodstream after injection. It is injected intramuscularly and metabolized similarly to testosterone, with 5α-reductase converting it to 3-norandrosterone (2). For humans, 0.4 mg/kg/day of nandrolone deconate is the approved therapeutic dose (3). A number of harmful side effects, both acute and chronic, can be brought on by consuming it (4). The activity of anabolic androgen steroids targets numerous organs and systems. Therefore, anabolic androgenic steroids may have negative effects on the immune system, cardiovascular, cerebrovascular, hepatic, musculoskeletal, endocrine, renal, and hematological systems (5-8). According to reports, anabolic androgenic steroids can improve the degree of protein synthesis during recovery and protect muscles from damage by increasing their capacity to withstand loading (9). This can lead to an improvement in tolerance to exercise. Anabolic androgenic steroids are being used illegally in large quantities by power sports athletes, such as bodybuilders and weightlifters, in an effort to gain more muscle mass and enhance their overall performance (10). Anabolic androgenic steroids are also abused by non-athletes. The Anabolic Steroids Control Act of 1990 has designated nandrolone decanoate injection as a Schedule III controlled substance (11). Most sporting organizations prohibit the non-medical use of anabolic androgenic steroids due to the significant health concerns involved. Additionally, the World Anti-Doping Agency (WADA) lists anabolic androgenic steroids (12). The misuse of these medications has grown to be a serious health issue (13). The Designer Steroid Control Act was passed in 2014 in an effort to plug the gaps for somewhat altered substances. These events fueled a massive demand for black-market products, facilitating the establishment of underground laboratories and the importation of pharmaceuticals made in countries with liberal anabolic androgenic steroid legislation (14). According to a poll, those who use anabolic androgen steroids usually don't tell their doctor that they use them and frequently have little faith in their expertise of these drugs (15). The global prevalence of Anabolic-Androgenic Steroid (AAS) use is believed to be between 1% and 5% (16). Male gender and/or athletic ability were found to be significant predictors of AAS misuse in a meta-analysis of 187 studies. The frequency is 1.6% in females and 6.4% in males (17). The cardiovascular risk associated with AAS comprises of the following: hypertension, life-threatening arrhythmia, myocardial dysfunction, coronary atherosclerosis (18), hypercoagulopathy, and hepatic dysfunction (19). In long-term steroid users, even after stopping AAS, concentrated left ventricular hypertrophy is frequently observed (21). This experimental research aims to investigate the impact of nandrolone decanoate administration on heart in white male albino rat models.
Materials & Methods
In this experimental study, injection of nandrolone decanoate for the procedure was procured from the market under the brand name ProtadecR 25 injection, Peanut oil.
The Wistar Albino rats served as experimental animals. The present study was conducted on a total of 20 adult male Albino rats of Wistar strain weighing 180-200 grams. The rats were procured from the Central Animal House of Government Medical College, Srinagar. Necessary clearance for the use of animals was obtained from Animal Institutional Ethical Committee constituted for this purpose.
Experimental Design:
A total of 20 male Albino rats were divided into 2 groups. Group A: Five rats served as Control group received an injection of 90% peanut oil. Group B: Fifteen rats received intramuscular decanoate at 10mg/kg body weight per week for 8 weeks. The animals were sacrificed and dissected after 8th week.
The animals were anesthetized by chloroform inhalation, as per the guidelines laid down by the “Committee for Purpose of Control and Supervision of Experiment on Animals”. After sacrificing, the rats were dissected.
Histological techniques:
These tissues were processed manually for block making using standard Histological techniques. Sections measuring 5-7 micrometers will be cut and fixed on glass slides. These sections were stained with Hematoxylin and Eosin. The Microscopic observations were recorded group wise using Light Microscope. Appropriate photographs were taken using Photographic Microscopy, labeled properly.
Results
Experimental groups were medicated with intramuscular injection of 10mg/kg body weight of nandrolone decanoate once a week, for 8 weeks respectively while Group A (Control) received injection of 90% peanut oil. The rats were sacrificed from each group at the end of the 8th week.
The animals were anesthetized by chloroform inhalation as per the guidelines laid down by the Committee. The dissection of rats was done under controlled conditions in the Animal House of GMC Srinagar with the help of the esteemed staff. A room set aside for the dissection of experimental animals was available at the Animal House, and all the equipment necessary for dissection was provided.
.JPG)
Gross examination of the rats was done, weight, activity, feeding, condition of the skin and presence of any external parasites were noted. All orifices were examined for the presence of discharges and any lesions either palpable or visible on the surface of the body were recorded accordingly. The Heart was identified, dissected out, cleaned, and put in containers containing formaldehyde. The gross morphology was noted and processed manually for block-making using standard histological techniques. Sections measuring 5-7 micrometers were cut and fixed on glass slides. These sections were stained with hematoxylin and eosin. The microscopic observations were recorded group-wise using a light microscope. Appropriate photographs were taken using photographic microscopy and labeled properly.
Results After the 8th week, on general examination, the animals were apparently healthy, alert, feeding well, and with no hair loss; however; their size and total body weight were increased.
Control Group [Group A]:
Microscopic features: On microscopic examination the basic architecture of the Heart was found to be preserved. No significant histological changes were seen in these animals as shown in (Figure 1)
Nandrolone Decanoate [Group B]: (10mg/kg body weight per week)
Microscopic features: Microscopic examination revealed intermuscular hemorrhages as shown in (Figures 2 and 3), congested myocardial vessels as shown in (Figure 4), widely separated cardiomyocytes and fragmented muscle fibers (Figure 5) and cardiomyocytes showing vesicular nuclei and few degenerative changes (Figure 6).
Nandrolone deconates are a group of synthetic derivative of testosterone that are used to maximize anabolic effects and minimize androgenic ones are known as anabolic androgenic steroids (1). Nandrolone decanoate has a half-life of six days, and is gradually released into the bloodstream after injection. It is injected intramuscularly and metabolized similarly to testosterone, with 5α-reductase converting it to 3-norandrosterone (2). For humans, 0.4 mg/kg/day of nandrolone deconate is the approved therapeutic dose (3). A number of harmful side effects, both acute and chronic, can be brought on by consuming it (4). The activity of anabolic androgen steroids targets numerous organs and systems. Therefore, anabolic androgenic steroids may have negative effects on the immune system, cardiovascular, cerebrovascular, hepatic, musculoskeletal, endocrine, renal, and hematological systems (5-8). According to reports, anabolic androgenic steroids can improve the degree of protein synthesis during recovery and protect muscles from damage by increasing their capacity to withstand loading (9). This can lead to an improvement in tolerance to exercise. Anabolic androgenic steroids are being used illegally in large quantities by power sports athletes, such as bodybuilders and weightlifters, in an effort to gain more muscle mass and enhance their overall performance (10). Anabolic androgenic steroids are also abused by non-athletes. The Anabolic Steroids Control Act of 1990 has designated nandrolone decanoate injection as a Schedule III controlled substance (11). Most sporting organizations prohibit the non-medical use of anabolic androgenic steroids due to the significant health concerns involved. Additionally, the World Anti-Doping Agency (WADA) lists anabolic androgenic steroids (12). The misuse of these medications has grown to be a serious health issue (13). The Designer Steroid Control Act was passed in 2014 in an effort to plug the gaps for somewhat altered substances. These events fueled a massive demand for black-market products, facilitating the establishment of underground laboratories and the importation of pharmaceuticals made in countries with liberal anabolic androgenic steroid legislation (14). According to a poll, those who use anabolic androgen steroids usually don't tell their doctor that they use them and frequently have little faith in their expertise of these drugs (15). The global prevalence of Anabolic-Androgenic Steroid (AAS) use is believed to be between 1% and 5% (16). Male gender and/or athletic ability were found to be significant predictors of AAS misuse in a meta-analysis of 187 studies. The frequency is 1.6% in females and 6.4% in males (17). The cardiovascular risk associated with AAS comprises of the following: hypertension, life-threatening arrhythmia, myocardial dysfunction, coronary atherosclerosis (18), hypercoagulopathy, and hepatic dysfunction (19). In long-term steroid users, even after stopping AAS, concentrated left ventricular hypertrophy is frequently observed (21). This experimental research aims to investigate the impact of nandrolone decanoate administration on heart in white male albino rat models.
Materials & Methods
In this experimental study, injection of nandrolone decanoate for the procedure was procured from the market under the brand name ProtadecR 25 injection, Peanut oil.
The Wistar Albino rats served as experimental animals. The present study was conducted on a total of 20 adult male Albino rats of Wistar strain weighing 180-200 grams. The rats were procured from the Central Animal House of Government Medical College, Srinagar. Necessary clearance for the use of animals was obtained from Animal Institutional Ethical Committee constituted for this purpose.
Experimental Design:
A total of 20 male Albino rats were divided into 2 groups. Group A: Five rats served as Control group received an injection of 90% peanut oil. Group B: Fifteen rats received intramuscular decanoate at 10mg/kg body weight per week for 8 weeks. The animals were sacrificed and dissected after 8th week.
The animals were anesthetized by chloroform inhalation, as per the guidelines laid down by the “Committee for Purpose of Control and Supervision of Experiment on Animals”. After sacrificing, the rats were dissected.
Histological techniques:
These tissues were processed manually for block making using standard Histological techniques. Sections measuring 5-7 micrometers will be cut and fixed on glass slides. These sections were stained with Hematoxylin and Eosin. The Microscopic observations were recorded group wise using Light Microscope. Appropriate photographs were taken using Photographic Microscopy, labeled properly.
Results
Experimental groups were medicated with intramuscular injection of 10mg/kg body weight of nandrolone decanoate once a week, for 8 weeks respectively while Group A (Control) received injection of 90% peanut oil. The rats were sacrificed from each group at the end of the 8th week.
The animals were anesthetized by chloroform inhalation as per the guidelines laid down by the Committee. The dissection of rats was done under controlled conditions in the Animal House of GMC Srinagar with the help of the esteemed staff. A room set aside for the dissection of experimental animals was available at the Animal House, and all the equipment necessary for dissection was provided.
.JPG)
Gross examination of the rats was done, weight, activity, feeding, condition of the skin and presence of any external parasites were noted. All orifices were examined for the presence of discharges and any lesions either palpable or visible on the surface of the body were recorded accordingly. The Heart was identified, dissected out, cleaned, and put in containers containing formaldehyde. The gross morphology was noted and processed manually for block-making using standard histological techniques. Sections measuring 5-7 micrometers were cut and fixed on glass slides. These sections were stained with hematoxylin and eosin. The microscopic observations were recorded group-wise using a light microscope. Appropriate photographs were taken using photographic microscopy and labeled properly.
Results After the 8th week, on general examination, the animals were apparently healthy, alert, feeding well, and with no hair loss; however; their size and total body weight were increased.
Control Group [Group A]:
Microscopic features: On microscopic examination the basic architecture of the Heart was found to be preserved. No significant histological changes were seen in these animals as shown in (Figure 1)
Nandrolone Decanoate [Group B]: (10mg/kg body weight per week)
Microscopic features: Microscopic examination revealed intermuscular hemorrhages as shown in (Figures 2 and 3), congested myocardial vessels as shown in (Figure 4), widely separated cardiomyocytes and fragmented muscle fibers (Figure 5) and cardiomyocytes showing vesicular nuclei and few degenerative changes (Figure 6).
Fig. 1. Photomicrograph of cardiac tissue of the rats from group B showing normal features at the end of the 8th week
Stain: H & E Magnification: 20x
.png)
Fig. 2. Photomicrograph of heart from group B showing intermuscular hemorrhage (black arrows) at the end of the 8th week.Stain: H & E Magnification: 10x
.png)
Fig. 3. Photomicrograph of heart from group B showing intermuscular hemorrhage (Black Arrow) at the end of 8th week.
Stain: H & E Magnifcation: 40x
.png)
Fig. 4. Photomicrograph of heart from group B showing congested blood vessel (Black arrow) in myocardium at the end of 8th week.
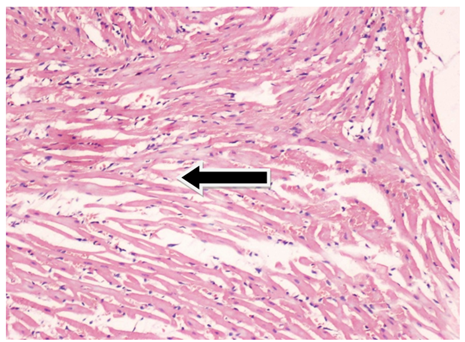
Stain: H & E Magnification: 20x
Fig. 5. Photomicrograph of heart from group B showing widely separated cardiac myocytes and fragmented muscle fibers (Black arrow) at the end of the 8th week of study.
Stain: H & E Magnification: 10x
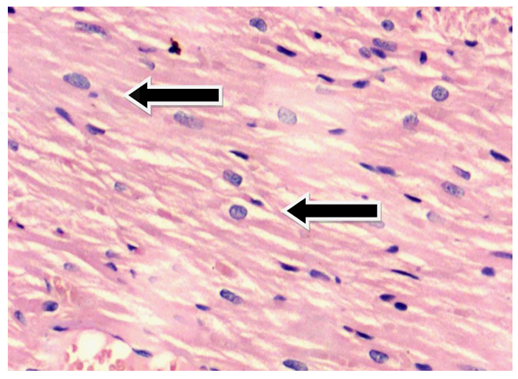
Fig. 6. Photomicrograph of heart from group B showing cardiomyocytes showing vesicular nuclei and few degenerative changes (Black arrow) in myocardium at the end of the 8th week.
Stain: H & E Magnification: 20x
DiscussionStain: H & E Magnification: 20x
.png)
.png)
Fig. 3. Photomicrograph of heart from group B showing intermuscular hemorrhage (Black Arrow) at the end of 8th week.
Stain: H & E Magnifcation: 40x
.png)
Fig. 4. Photomicrograph of heart from group B showing congested blood vessel (Black arrow) in myocardium at the end of 8th week.

Stain: H & E Magnification: 20x
Fig. 5. Photomicrograph of heart from group B showing widely separated cardiac myocytes and fragmented muscle fibers (Black arrow) at the end of the 8th week of study.
Stain: H & E Magnification: 10x

Fig. 6. Photomicrograph of heart from group B showing cardiomyocytes showing vesicular nuclei and few degenerative changes (Black arrow) in myocardium at the end of the 8th week.
Stain: H & E Magnification: 20x
In the present study, no apparent gross abnormality was noted. Light microscopy of cardiac tissue revealed normal histology of the heart in Group A (Control). Group B (ND) at the end of the 8thweek, shows intermuscular hemorrhages, congestion of myocardial vessels, separated cardiacmyocytes and fragmented muscle fibers, vesicular nuclei and severe degenerative changes.
Ren (22) showed that steroids alone can induce cardiomyocyte hypertrophy a dose much higher than that used in our study (15%) increase with nandrolone alone. The divergence may be the result of different study design (e.g., their study was performed in cell culture conditions); therefore, the doses of the hormones may not be comparable.
Abdelhafez (23) showed highly degenerated muscle fibers with areas of hemorrhage and widened endomysium. Also, she demonstrated a numerous pyknotic and karyolytic nuclei.
The same findings were obtained by Soliman (24) who found that nandrolone administration causes a 10-fold increase in heart collagen.
Parssinen (21) added that this effect tended to be dose-dependent. These short-term changes in collagen metabolism may be explained by increased anabolic effects in muscle. The same results were obtained by Soliman (24).
Also, Elgendy (25) reported hypertrophy and degeneration of both cardiac and skeletal muscles and explained this by its effect on the androgen receptors that are widely distributed in different types of muscles.
Ren (22) showed that steroids alone can induce cardiomyocyte hypertrophy a dose much higher than that used in our study (15%) increase with nandrolone alone. The divergence may be the result of different study design (e.g., their study was performed in cell culture conditions); therefore, the doses of the hormones may not be comparable.
Conclusion
According to this study, using nandrolone can cause cardiac abnormalities like severe degenerative changes, myocardial vascular congestion, and intermuscular hemorrhages. These findings are consistent with earlier research, which has suggested increased collagen and muscle fiber degeneration. As the main limitation of the current study was low study population, the study's findings highlight and recommended the significance of future research into the long-term effects of these medications and effects of toxic doses of nandrolone, as well as careful consideration of study design and dose.
Acknowledgments
We would like to thank the Faculty of the Postgraduate Department of Anatomy, Government Medical College Srinagar, India and all the scholars whose articles are cited and included in references.
Ethical statement
This study was conducted after approval by the ethics committee of Government Medical College, Srinagar with code of ethics MC-421 (GMCS-2020).
Data availability
The raw data supporting the conclusions of this article are available from the authors upon reasonable request.
Author contributions
All the authors contributed equally from study design, methodology, procurement of drug used, processing of tissues, results, discussion to final submission.
Funding/Support
None.
Conflict of interest
The authors have no conflict of interest in this study.
Type of Study: orginal article |
Subject:
Other
References
1. Evans NA. Current Concepts in Anabolic-Androgenic Steroids. Am J Sports Med 2004;32:534-42. [DOI:10.1177/0363546503262202] [PMID]
2. Pan MM, Kovac JR. Beyond testosterone cypionate: Evidence behind the use of nandrolone in male health and wellness. TranslAndrolUrol2016;5:213-9. [DOI:10.21037/tau.2016.03.03] [PMID] []
3. Tamaki T, Shiraishi T, Takeda H, Matsumiya T, Roy RR, Edgerton VR. Nandrolone Decanoate Enhances Hypothalamic Biogenic Amines in Rats. Med Sci Sports Exerc2003;35:32-8. [DOI:10.1097/00005768-200301000-00006] [PMID]
4. Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin Ther2001;23:1355-90. [DOI:10.1016/S0149-2918(01)80114-4] [PMID]
5. Turillazzi E, Perilli G, Di Paolo M, Neri M, Riezzo I, Fineschi V. Side effects of AAS abuse: An overview. Mini-Rev Med Chem 2011;11:374-89. [DOI:10.2174/138955711795445925] [PMID]
6. Van Amsterdam J, Opperhuizen A, Hartgens F. Adverse health effects of anabolic-androgenic steroids. Regul. ToxicolPharmacol 2010; 57:117-23. [DOI:10.1016/j.yrtph.2010.02.001] [PMID]
7. Brower KJ. Anabolic steroid abuse and dependence. Curr. Psychiatry Rep 2002;4: 377-87. [DOI:10.1007/s11920-002-0086-6] [PMID]
8. Basaria S. Androgen Abuse in Athletes: Detection and Consequences. J Clin Endocrinol Metab 2010; 95:1533-43. [DOI:10.1210/jc.2009-1579] [PMID]
9. Frati P, Busardò FP, Cipolloni L, De Dominicis E, Fineschi V. Anabolic Androgenic Steroid (AAS) Related Deaths: Autoptic, Histopathological and Toxicological Findings. CurrNeuropharmacol2015;13:146-59. [DOI:10.2174/1570159X13666141210225414] [PMID] []
10. Hall RC. Abuse of supraphysiologic doses of anabolic steroids. South Med J 2005;98:550-5. [DOI:10.1097/01.SMJ.0000157531.04472.B2] [PMID]
11. Dotson JL, Brown RT. The History of the Development of Anabolic- Androgenic Steroids. Pediatr. Clin N Am 2007;54:761-9. [DOI:10.1016/j.pcl.2007.04.003] [PMID]
12. Ostojic S, editor. Steroids: From Physiology to Clinical Medicine. BoD-Books on Demand; 2012 Nov 21; pp. 169-186. [DOI:10.5772/46119]
13. De Souza GL, Hallak J. Anabolic steroids and male infertility: A comprehensive review. BJU Int 2011;108:1860-5. [DOI:10.1111/j.1464-410X.2011.10131.x] [PMID]
14. Fink J, Schoenfeld BJ, Hackney AC, Matsumoto M, Maekawa T, Nakazato K, Horie S. Anabolic-androgenic steroids: Procurement and administration practices of doping athletes. Physician Sportsmed2018;47:10-4. [DOI:10.1080/00913847.2018.1526626] [PMID]
15. Pope HG, Kanayama G, Ionescu-Pioggia M, Hudson JI. Anabolic steroidusers'attitudes towards physicians. Addiction 2004;99:1189- 94. [DOI:10.1111/j.1360-0443.2004.00781.x] [PMID]
16. Anawalt BD. Diagnosis and Management of Anabolic Androgenic Steroid Use. J Clin Endocrinol Metab. 2019 Jul 1;104(7):2490-2500. doi: 10.1210/jc.2018-01882. [DOI:10.1210/jc.2018-01882] [PMID] []
17. Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014 May;24(5):383-98. doi: 10.1016/j.annepidem.2014.01.009. [DOI:10.1016/j.annepidem.2014.01.009] [PMID]
18. Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, Hoffmann U, Pope HG Jr. Cardiovascular Toxicity of Illicit Anabolic-Androgenic Steroid Use. Circulation. 2017 May 23;135(21):1991-2002. doi: 10.1161/CIRCULATIONAHA.116.026945. [DOI:10.1161/CIRCULATIONAHA.116.026945] [PMID] []
19. Kam PC, Yarrow M. Anabolic steroid abuse: physiological and anaesthetic considerations. Anaesthesia. 2005 Jul;60(7):685-92. doi: 10.1111/j.1365-2044.2005.04218.x. PMID: 15960720. [DOI:10.1111/j.1365-2044.2005.04218.x] [PMID]
20. Vanberg P, Atar D. Androgenic anabolic steroid abuse and the cardiovascular system. Handb Exp Pharmacol. 2010;(195):411-57. doi: 10.1007/978-3-540-79088-4_18. [DOI:10.1007/978-3-540-79088-4_18] [PMID]
21. Parssinen M, Karila T, Kovanen V, Seppala T. The effect of supraphysiological doses of anabolic androgenic steroids on collagen metabolism. Int J Sports Med 2000;21:406-11. [DOI:10.1055/s-2000-3834] [PMID]
22. Ren R, Oakley RH, Cruz-Topete D, Cidlowski JA. Dual role for glucocorticoids in cardio- myocyte hypertrophy and apoptosis. Endocrinol- ogy 2012;153(11):5346-60. [DOI:10.1210/en.2012-1563] [PMID] []
23. Abdelhafez HM. Histological, histochemical and ultrastructural studies on the effect of Deca-Durabolin and whey protein isolate on cardiac muscle in adult male albino rats. Int J Adv Res 2014;2(10):164-87 [Google Scholar]
24. Soliman ME, El-Saify GS, Badawy KNS, Soliman MAM, Abo-Habsa SS. Effect of nandrolone on rat cardiac muscle and the possible protective role of Vitamin E: A light and electron microscopic study. J Am Sci 2017;13. [URL]
25. Elgendy HAE, Alhawary AAE, El-Shahat MAE, Ali AT. Effect of anabolic ster- oids on the cardiac and skeletal muscles of adult male rats. Int J Clin Dev Anat 2018;4(1):1. [DOI:10.11648/j.ijcda.20180401.11]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







 gmail.com
gmail.com