Volume 10, Issue 3 (7-2024)
Journal of Research in Applied and Basic Medical Sciences 2024, 10(3): 213-223 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Suleiman Jada M, Umar Y, Usman Wurochekke A. Antiplasmodial activity of Azanza Garckeana root bark extract and its effect on hematological indices in plasmoduim berghei infected mice. Journal of Research in Applied and Basic Medical Sciences 2024; 10 (3) :213-223
URL: http://ijrabms.umsu.ac.ir/article-1-326-en.html
URL: http://ijrabms.umsu.ac.ir/article-1-326-en.html
Department of Biochemistry, Faculty of Life Sciences, Modibbo Adama University, Yola, Adamawa state, Nigeria , jadasm84@gmail.com
Full-Text [PDF 427 kb]
(685 Downloads)
| Abstract (HTML) (1247 Views)
Hematological Analysis:
Hematological indices, using a hematology analyzer called HumaReader Plus (HUMAN Diagnostic Worldwide, Wiesbaden, Germany), the levels of packed cell volume (PCV), hemoglobin (Hb), red blood cells (RBC), and white blood cells (WBC) were measured.
Determination of Parasitaemia Level and Chemo-suppression activity:
Parasitaemia levels were assessed on four different days following the start of the treatment. To determine this, a small sample of blood from each animal's tail was placed on glass slides and left to dry at air. The slides were then treated with methanol, stained using Giemsa, and examined using a 100x objective lens under a microscope. The count of red blood cells infected with parasites observed on each slide was recorded. This count was used in the formula below to calculate the parasitaemia level.

Where; RBC= Red Blood Cells and PRBC= Parasitized Red Blood Cells.
Comparing the extracts to the untreated control, the percentage of parasitaemia suppression was calculated. This computation was made using a particular formula that Dikasso et al. (19) detailed. This formula was used to measure the level of parasitaemia reduction that the extracts were able to accomplish.
Table 1. Phytochemical constituent of Azanza garckeana root-bark extract.
Key: Absent (–); Present (+).
Parasitaemia and Chemosuppression Level of Treated P. berghei Infected Mice
Figure 1 shows how the 4-day suppressive test with A. garckeana root-bark extract affected the parasitaemia levels in P. berghei-infected mice. The root-bark extract of A. garckeana showed a dosage-dependent reduction in parasitaemia levels that was both quick and significant, with the dose of 300 mg/kg showing the greatest drop.
.jpg)
Fig. 1. Effects of Azanza garckeana root-bark extract on parasitaemia level in Plasmodium berghei-infected mice
aP<0.05 lower compared to negative control, bP<0.05 lower compared to Artemether, cP<0.05 higher compared to Artemether, dP<0.05 higher compared to normal control. AZRE: Azanza garckeana root-bark extract.
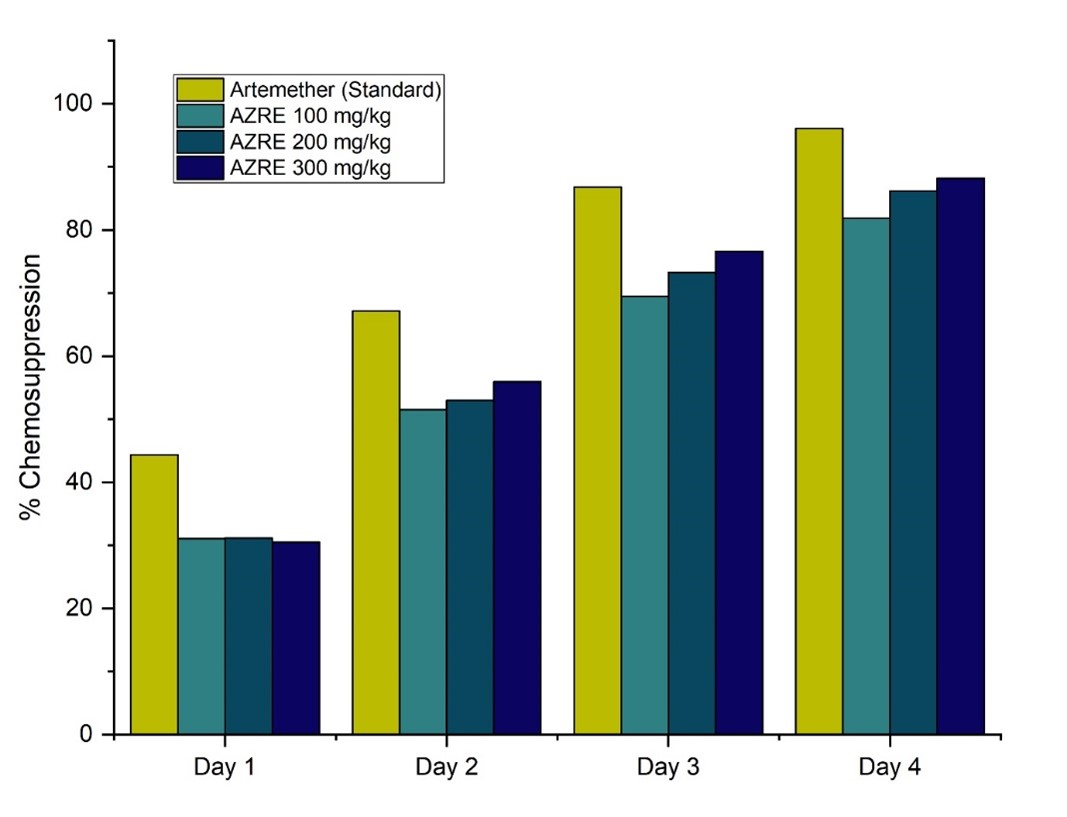
Fig. 2. Effects of Azanza garckeana root-bark extract on chemosuppression level of Plasmodium berghei infected mice
Table 2. Effects of A. garckeana root-bark extract on the hematological level of P. berghei infected mice after the 4-day suppressive test
Values are expressed as Mean ± S.E.M, n=5;
aP < 0.05 higher compared to negative control; bP < 0.05 higher compared to normal control; cP < 0.05 lower compared to negative control; dP < 0.05 lower compared to normal control; eP < 0.05 higher compared to Artemether; fP < 0.05 lower compared to Artemether. RBC: Red blood cells, WBC: White blood cells, PLT: Platelets, PCV: Packed cell volume, HGB: Hemoglobin MCV: Mean corpuscular volume, MHC: Mean hemoglobin concentration, LYM: Lymphocytes, NEU: Neutrophils, BAS: Basophils, MCHC: Mean corpuscular hemoglobin concentration; AZRE: Azanza garckeana root-bark extract.
Discussion
Phytochemical Composition of the Root-bark Extract of Azanza garckeana:
The phytochemical screening of the aqueous extract of A. garckeana root bark revealed the presence of various bioactive compounds, aligning with previous studies on different parts of the plant (8,9,10). The identified phytochemicals, including saponins, tannins, flavonoids, alkaloids, glycosides, phenols, and steroids, provide a foundation for understanding the potential therapeutic effects of the root-bark extract against P. berghei in mice.
Parasitaemia and Chemosuppression Level of Treated P. berghei Infected Mice:
The 4-day suppressive test results on the effects of A. garckeana root-bark extract on the parasitaemia level of P. berghei-infected mice revealed promising outcomes, suggesting its significant antiplasmodial properties as a potential therapeutic agent for malaria treatment. In comparison to the negative control, which exhibited a steady increase in parasitaemia levels, and the artemether group, where parasitaemia levels decreased, the A. garckeana root-bark extract demonstrated a rapid and substantial significant reduction in parasitaemia levels but not surpassing the efficacy of artemether. The dose-dependent nature of the antiplasmodial activity was evident, with the 300 mg/kg dose exhibiting the most significant decrease in parasitaemia levels, aligning with previous studies highlighting the dose-dependent antiplasmodial effects of A. garckeana (20). These findings underscore the potential of A garckeana as a promising and dose-responsive alternative therapy for malaria (20).
The results of the 4-day suppressive test evaluating the effects of A. garckeana root-bark extract on the chemosuppression level in P. berghei-infected mice revealed a dose-dependent response. The negative control exhibited no chemosuppression, serving as a baseline for uncontrolled parasitemia growth. In comparison, the standard antimalarial drug, artemether, demonstrated a substantial increase in chemosuppression over the treatment period, reaching an impressive 96.09% on day 4. Notably, the A. garckeana root-bark extract groups at various concentrations (100 mg/kg, 200 mg/kg, and 300 mg/kg) exhibited dose-dependent chemosuppressive effects. The highest dose, 300 mg/kg, demonstrated a significant chemosuppression level of 88.17% on day 4, approaching the efficacy of artemether. These findings show the ability of A. garckeana to impede the development of P. berghei, indicating its potential utility as an antimalarial agent. This is consistent with the findings of a study on the antimalarial activity of Zingiber Officinale and Echinops Kebericho (21). Similar results were obtained by another study when using Salvadora persica root extract and Balanites rotundifolia leaf extract to prevent P. berghei growth (22).
The aqueous root-bark extract of A. garckeana demonstrated appreciable and dose-dependent chemosuppressive effects against P. berghei infection in mice. The identified phytochemicals, including flavonoids, saponins and alkaloids, likely mediate antiparasitic effects through well-established mechanisms of cell membrane disruption, protein binding, and heme polymerization to exert parasite clearance (23, 24). These multimodal actions help circumvent current drug resistance issues. About 88% suppression at 300 mg/kg is therapeutically relevant, considering prior studies demonstrating >60% efficacy as a cut-off for antimalarial potential (24).
The observed reduction in malaria parasite growth by the A. garckeana root-bark extract may occur in different ways that we are not fully aware of. It could enhance the immune system indirectly or block certain pathways in the body. The plant contains phytosteroids, phenolic compounds, and flavonoids, which are known for their potential to boost the immune system, reduce inflammation, and act as antioxidants. Additionally, these plant components might interact with the known factors involved in the development and life cycle of the malaria parasite, using a similar or unique method (26).
In this study, it was found that a high dose of the plant extract was effective against the parasite. By nanoformulating the extract, the same therapeutic outcomes may be achieved with lower doses, minimizing toxicity and improving patient compliance. Nanoformulation involves encapsulating active compounds into nanoparticles to enhance their effectiveness and reduce dosage requirements (27). This enhances the extract's solubility, stability, and targeted delivery, leading to better bioavailability and therapeutic outcomes. Additionally, it allows for controlled release kinetics, optimizing treatment strategies (27). Overall, nanoformulation of medicinal plant extracts shows promise for improving antimalarial therapies and herbal medicine in general (28).
Hematological Level:
The hematological analysis following the 4-day suppressive test with A. garckeana root-bark extract on P. berghei-infected mice reveals notable impacts on various blood parameters. In comparison to the naive (non-infected) group, the negative control group, representing untreated infected mice, exhibited significant reductions in key parameters, including white blood cells (WBC), red blood cells (RBC), platelets (PLT), packed cell volume (PCV), and hemoglobin (HGB). These reductions align with the expected effects of P. berghei infection on blood components. Artemether, the standard antimalarial drug, showed some restoration of these parameters, indicating its efficacy in mitigating malaria-induced hematological alterations. Interestingly, A. garckeana at different doses demonstrated varying effects on the hematological parameters which concise other findings on Ocimum gratissimum (29) and Jatropha tanjorensis leaf (30). The 300 mg/kg dose of A. garckeana showed an increase in WBC, RBC, PLT, PCV, and HGB compared to the negative control, suggesting a potential protective effect against malaria-induced hematological changes. However, some parameters, such as mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and lymphocytes (LYM), showed mixed responses across A. garckeana groups. These findings suggest that A. garckeana root-bark extract may have a modulatory effect on hematological parameters in P. berghei-infected mice, with the 300 mg/kg dose demonstrating more pronounced positive effects.
The study revealed that administering an aqueous root-bark extract of A. garckeana to mice infected with P. berghei resulted in a significant increase in white blood cells (WBC) and neutrophil (NEU) counts when compared to the uninfected control group. With 100 and 200 mg/kg A. garckeana compared to naïve mice, basophil percentages were similarly increased.
White blood cells play an important role in the immune response against malarial infection. Specifically, neutrophils are involved in the initial innate response and help control parasite multiplication through phagocytosis and cytokine release (31). The observed increase in WBCs and neutrophils suggests that A. garckeana extract may stimulate immune cell production and activity, enhancing the clearance of parasitized erythrocytes. This immune-boosting effect could contribute to the antimalarial efficacy noted in prior in vivo studies testing extracts of this plant (26).
Elevations in circulating basophils were also seen with 100 and 200 mg/kg A. garckeana extract treatment. Though the function of basophils in malaria is less clear, some evidence suggests they may play a regulatory role through interactions with T-cells and the release of IL-4 (32). The higher percentages could reflect increased activation and recruitment related to the extract's influence on immune processes during infection.
No significant alterations were detected between groups for other hematological indices like red blood cells, hemoglobin, platelets, or red cell volume and morphology. The lack of major changes indicates the extract does not cause haematoxicity and is well-tolerated at the tested doses. Artemether similarly did no effect on these measures at its curative concentration.
Conclusion
The medicinal plant A. garckeana's aqueous root-bark extract showed strong antiplasmodial action against P. berghei infection in mice. Significant chemosuppression was attained, and hematological parameters were modulated by it. The findings confirm the ethnomedical significance of A. garckeana and encourage more research into the phytochemical components of the plant to develop antimalarial medications. However, limited access to advanced analytical tools like gas chromatography and mass spectrometry hindered detailed characterization of the plant's active compounds. Also, the majority of previous studies that sought to confirm the antimalarial properties of plants employed methodologies akin to ours. Despite this, the plant's antimalarial potential warrants further exploration, particularly in isolating and characterizing its active compounds. Additionally, exploring nanoformulation of the plant extract could offer promising avenues to enhance its therapeutic benefits and reduce dosage requirements.
Acknowledgments
The authors sincerely appreciate Biochemistry Department of Modibbo Adama University, Yola for allowing them to use the departmental laboratory and other necessary facilities for the success of this study.
Ethical Statement
The Animal Ethics Committee at the Department of Animal Science, Faculty of Agricultural Science, Modibbo Adama University, Yola, gave the clearance and authorization for the use of animals in this study (MAU/FAS/AEC/AS/2023/036).
Data availability
The supporting data of this study is always obtainable on request from the corresponding author.
Authors’ contributions
Author MSJ designed the study, conducted the statistical analysis and wrote the first draft of the manuscript; author YU performed the experiments; author AUW managed the analyses of the study and literature searches. All authors read and approved the final manuscript.
Funding/Support
No funding support received for this work. The work is fully funded by the authors.
Conflict of interest
The authors have no conflict of interest in this study.
Full-Text: (211 Views)
Introduction
Malaria represents a severe global health burden, particularly affecting vulnerable populations in sub-Saharan Africa, including children under the age of five and pregnant women (1). With a predicted 249 million cases in 85 malaria-endemic countries in 2022, the number of malaria cases worldwide topped pre-COVID-19 levels, according to the WHO's 2023 World Malaria Report. The number of threats posed by climate change to global response is 55% more than anticipated for the 2025 Global Technical Strategy (2).
The growing risk of drug resistance imperils the effectiveness of existing antimalarial medications, including Artemisinin-based combination treatments (ACTs) (3). Drug-resistant parasite strains have emerged as a result of extended exposure to antimalarial medications; this phenomenon has been seen most prominently in South-East Asia, where ACT-resistant P. falciparum parasites impede the clearance of parasites (4). This growing resistance necessitates urgent efforts to develop novel antimalarial products.
Traditional medicinal plants, known for their safety and efficacy, have been a valuable source of drug discovery. About 94 plant species have yielded 122 drugs, emphasizing the importance of ethno botanical leads (5). Azanza garckeana, holds promise as a medicinal plant, grown exclusively in Tula village with the coordinates 9.83333, 11.46667, Gombe State of Nigeria. With a rich history of use in Northern Nigeria for over 20 human diseases, including cough, chest pains, infertility, and sexually transmitted infections, A. garckeana has been recognized for its diverse bioactive metabolites (6).
The purpose of this study was to assess the antimalarial efficacy of an aqueous extract of A. garckeana's root bark in mice harboring a Plasmodium berghei infection. Considering the high rates of morbidity and death in sub-Saharan Africa, particularly among vulnerable groups (7), the decision was motivated by the pressing need for innovative therapies for malaria. Traditional medicinal plants like A. garckeana, with their historical efficacy, offer a potential avenue for novel treatments (8). By exploring the antimalarial potential of A. garckeana's root-bark extract, this study aims to contribute to the discovery of effective malaria treatments.
Despite the introduction of Artemisinin, mefloquine, sulphadoxine, and pyrimethamine in Nigeria, significant drug resistance against Plasmodium falciparum persists, highlighting the need for alternative treatments (11). The emergence of resistance in Artemisia, the latest plant used in malaria treatment, adds urgency to the situation (12). The declining efficacy of ACTs in regions with a history of antimalarial drug resistance, like the Thai-Cambodia border, underscores the global challenge posed by drug-resistant strains of P. falciparum (13). As drug-resistant parasites continue to spread, the development of effective control measures becomes imperative (14). In Nigeria, where accessibility to pharmaceutical drugs is often limited, traditional remedies, including A. garckeana, offer a potential alternative that is easily accessible (15). This study aims to investigate the antiplasmodial activities of A. garckeana and its effect on hematological parameters, contributing to the development of affordable and accessible antimalarial agents in Nigeria.
Materials & Methods
Plant Material:
A fresh root bark of A. garckeana was collected from Gombe State and placed in a clean plastic bag. The leaves were collected for identification by a taxonomist in the Department of Plant Sciences, Modibbo Adama University, Yola.
Preparation of the Plant Material:
The root bark of A. garckeana was cleaned with water and rinsed with distilled water to remove mud and dirt. After that, it was gently spread out on a spotless surface and left for two weeks to air dry at room temperature. The dried root bark was then ground into a homogeneous powder using an electric mill. In preparation for a subsequent aqueous extraction, the powder was kept in sachets in a dry, ventilated cabinet out of direct sunlight.
Extraction of Plant Material:
The maceration process, as outlined by Bouratoua et al. (16), was used for extracting the plant material. In summary, one litter of distilled water was used to soak 100 g of A. garckeana root-bark powder, which was periodically agitated and remained at room temperature for 72 hours. The weight of the powder was accurately determined using an analytical balance. Next, a Whatman filter paper was used to filter the mixture. After 48 hours in a tray, the filtrate was turned into a dry powder. Weighed and placed into airtight containers, the powdered extract was sealed and stored at 40 °C until it was analyzed. The extract's percentage yield was evaluated using the weight-by-weight (%w/w) method.
Phytochemical Screening of Root-bark Extracts of Azanza garckeana:
The conventional qualitative techniques used by Trease and Evans (17) were utilized to identify the phytochemical elements of root bark A. garckeana.
Laboratory Animals:
The study included thirty (30) mice weighing between 20 and 30 grams at 4 weeks of age, which were obtained from the National Veterinary Research Institute located in Vom, Plateau State, Nigeria. They received water and rodent pellets to eat. Before being employed, two weeks were allowed for acclimation to the experimental room temperature of 25°C and twelve hours of daylight. When dealing with the animals, all ethical protocols and recommendations were followed (18).
Parasite:
Plasmodium berghei, a strain susceptible to Artemether, was acquired from the National Veterinary Research Institute in Lagos State.
Parasite Inoculation:
Through cardiac plexus puncture, parasitized erythrocytes were removed from donor animals and diluted with trisodium citrate. On day 0, mice received an intraperitoneal injection of 0.2 ml blood solution containing 106–107 parasitized erythrocytes.
Experimental Design and Animal Grouping:
Thirty (30) mice were separated into six equal groups (Groups A through E) at random, each with five mice, the mice were then given the following treatment.
Malaria represents a severe global health burden, particularly affecting vulnerable populations in sub-Saharan Africa, including children under the age of five and pregnant women (1). With a predicted 249 million cases in 85 malaria-endemic countries in 2022, the number of malaria cases worldwide topped pre-COVID-19 levels, according to the WHO's 2023 World Malaria Report. The number of threats posed by climate change to global response is 55% more than anticipated for the 2025 Global Technical Strategy (2).
The growing risk of drug resistance imperils the effectiveness of existing antimalarial medications, including Artemisinin-based combination treatments (ACTs) (3). Drug-resistant parasite strains have emerged as a result of extended exposure to antimalarial medications; this phenomenon has been seen most prominently in South-East Asia, where ACT-resistant P. falciparum parasites impede the clearance of parasites (4). This growing resistance necessitates urgent efforts to develop novel antimalarial products.
Traditional medicinal plants, known for their safety and efficacy, have been a valuable source of drug discovery. About 94 plant species have yielded 122 drugs, emphasizing the importance of ethno botanical leads (5). Azanza garckeana, holds promise as a medicinal plant, grown exclusively in Tula village with the coordinates 9.83333, 11.46667, Gombe State of Nigeria. With a rich history of use in Northern Nigeria for over 20 human diseases, including cough, chest pains, infertility, and sexually transmitted infections, A. garckeana has been recognized for its diverse bioactive metabolites (6).
The purpose of this study was to assess the antimalarial efficacy of an aqueous extract of A. garckeana's root bark in mice harboring a Plasmodium berghei infection. Considering the high rates of morbidity and death in sub-Saharan Africa, particularly among vulnerable groups (7), the decision was motivated by the pressing need for innovative therapies for malaria. Traditional medicinal plants like A. garckeana, with their historical efficacy, offer a potential avenue for novel treatments (8). By exploring the antimalarial potential of A. garckeana's root-bark extract, this study aims to contribute to the discovery of effective malaria treatments.
Despite the introduction of Artemisinin, mefloquine, sulphadoxine, and pyrimethamine in Nigeria, significant drug resistance against Plasmodium falciparum persists, highlighting the need for alternative treatments (11). The emergence of resistance in Artemisia, the latest plant used in malaria treatment, adds urgency to the situation (12). The declining efficacy of ACTs in regions with a history of antimalarial drug resistance, like the Thai-Cambodia border, underscores the global challenge posed by drug-resistant strains of P. falciparum (13). As drug-resistant parasites continue to spread, the development of effective control measures becomes imperative (14). In Nigeria, where accessibility to pharmaceutical drugs is often limited, traditional remedies, including A. garckeana, offer a potential alternative that is easily accessible (15). This study aims to investigate the antiplasmodial activities of A. garckeana and its effect on hematological parameters, contributing to the development of affordable and accessible antimalarial agents in Nigeria.
Materials & Methods
Plant Material:
A fresh root bark of A. garckeana was collected from Gombe State and placed in a clean plastic bag. The leaves were collected for identification by a taxonomist in the Department of Plant Sciences, Modibbo Adama University, Yola.
Preparation of the Plant Material:
The root bark of A. garckeana was cleaned with water and rinsed with distilled water to remove mud and dirt. After that, it was gently spread out on a spotless surface and left for two weeks to air dry at room temperature. The dried root bark was then ground into a homogeneous powder using an electric mill. In preparation for a subsequent aqueous extraction, the powder was kept in sachets in a dry, ventilated cabinet out of direct sunlight.
Extraction of Plant Material:
The maceration process, as outlined by Bouratoua et al. (16), was used for extracting the plant material. In summary, one litter of distilled water was used to soak 100 g of A. garckeana root-bark powder, which was periodically agitated and remained at room temperature for 72 hours. The weight of the powder was accurately determined using an analytical balance. Next, a Whatman filter paper was used to filter the mixture. After 48 hours in a tray, the filtrate was turned into a dry powder. Weighed and placed into airtight containers, the powdered extract was sealed and stored at 40 °C until it was analyzed. The extract's percentage yield was evaluated using the weight-by-weight (%w/w) method.
Phytochemical Screening of Root-bark Extracts of Azanza garckeana:
The conventional qualitative techniques used by Trease and Evans (17) were utilized to identify the phytochemical elements of root bark A. garckeana.
Laboratory Animals:
The study included thirty (30) mice weighing between 20 and 30 grams at 4 weeks of age, which were obtained from the National Veterinary Research Institute located in Vom, Plateau State, Nigeria. They received water and rodent pellets to eat. Before being employed, two weeks were allowed for acclimation to the experimental room temperature of 25°C and twelve hours of daylight. When dealing with the animals, all ethical protocols and recommendations were followed (18).
Parasite:
Plasmodium berghei, a strain susceptible to Artemether, was acquired from the National Veterinary Research Institute in Lagos State.
Parasite Inoculation:
Through cardiac plexus puncture, parasitized erythrocytes were removed from donor animals and diluted with trisodium citrate. On day 0, mice received an intraperitoneal injection of 0.2 ml blood solution containing 106–107 parasitized erythrocytes.
Experimental Design and Animal Grouping:
Thirty (30) mice were separated into six equal groups (Groups A through E) at random, each with five mice, the mice were then given the following treatment.
| Group | Descriptions | Treatment |
| A | Naïve | No inoculation, no treatment |
| B | Negative control | Inoculation with P. berghei, no treatment |
| C | standard control (Artemether) | Inoculation with P. berghei + 10mg/kg of standard antimalarial drug (Artemether) |
| D | Treatment 1 | Inoculation with P. berghei + 100mg/kg aqueous extract of A. garckeana root bark |
| E | Treatment 2 | Inoculation with P. berghei + 200mg/kg aqueous extract of A. garckeana root bark |
| F | Treatment 3 | Inoculation with P. berghei + 300mg/kg aqueous extract of A. garckeana root bark. |
Hematological Analysis:
Hematological indices, using a hematology analyzer called HumaReader Plus (HUMAN Diagnostic Worldwide, Wiesbaden, Germany), the levels of packed cell volume (PCV), hemoglobin (Hb), red blood cells (RBC), and white blood cells (WBC) were measured.
Determination of Parasitaemia Level and Chemo-suppression activity:
Parasitaemia levels were assessed on four different days following the start of the treatment. To determine this, a small sample of blood from each animal's tail was placed on glass slides and left to dry at air. The slides were then treated with methanol, stained using Giemsa, and examined using a 100x objective lens under a microscope. The count of red blood cells infected with parasites observed on each slide was recorded. This count was used in the formula below to calculate the parasitaemia level.


Where; RBC= Red Blood Cells and PRBC= Parasitized Red Blood Cells.
Comparing the extracts to the untreated control, the percentage of parasitaemia suppression was calculated. This computation was made using a particular formula that Dikasso et al. (19) detailed. This formula was used to measure the level of parasitaemia reduction that the extracts were able to accomplish.
Data Analysis:
The data was presented as mean ± standard deviation. With SPSS Version 27, analysis for the in vivo task was accomplished. ANOVA, a one-way test of statistical significance, and Tukey's Honest Significant Difference post-hoc test was used to determine the difference in survival time and parasitaemia decrease within each class.
Results
Phytochemical Composition of the Root-bark Extract of Azanza garckeana:
Table 1 displays the phytochemical makeup of the A. garckeana root-bark extract. There were phytochemicals such as tannins, flavonoids, alkaloids, phenols, glycosides, and steroids but not terpenoids.
The data was presented as mean ± standard deviation. With SPSS Version 27, analysis for the in vivo task was accomplished. ANOVA, a one-way test of statistical significance, and Tukey's Honest Significant Difference post-hoc test was used to determine the difference in survival time and parasitaemia decrease within each class.
Results
Phytochemical Composition of the Root-bark Extract of Azanza garckeana:
Table 1 displays the phytochemical makeup of the A. garckeana root-bark extract. There were phytochemicals such as tannins, flavonoids, alkaloids, phenols, glycosides, and steroids but not terpenoids.
Table 1. Phytochemical constituent of Azanza garckeana root-bark extract.
| Phytochemical | Azanza garckeana |
| Saponins | + |
| Tannins | + |
| Flavonoids | + |
| Alkaloids | + |
| Glycosides | + |
| Phenols | + |
| Steroids | + |
| Terpenoids | – |
Parasitaemia and Chemosuppression Level of Treated P. berghei Infected Mice
Figure 1 shows how the 4-day suppressive test with A. garckeana root-bark extract affected the parasitaemia levels in P. berghei-infected mice. The root-bark extract of A. garckeana showed a dosage-dependent reduction in parasitaemia levels that was both quick and significant, with the dose of 300 mg/kg showing the greatest drop.
.jpg)
Fig. 1. Effects of Azanza garckeana root-bark extract on parasitaemia level in Plasmodium berghei-infected mice
aP<0.05 lower compared to negative control, bP<0.05 lower compared to Artemether, cP<0.05 higher compared to Artemether, dP<0.05 higher compared to normal control. AZRE: Azanza garckeana root-bark extract.
Figure 2 illustrates the impact of A. garckeana root-bark extract on the chemosuppression levels in P. berghei-infected mice during the 4-day suppressive test. Values represent Mean ± S.E.M for each group on Days 1 to 4. Treatment groups at different concentrations show varying degrees of chemosuppression. The 300 mg/kg dose shows the highest chemosuppresion on day-4 but below the standard control (Artemether).

Fig. 2. Effects of Azanza garckeana root-bark extract on chemosuppression level of Plasmodium berghei infected mice
Hematological Level:
Table 2 summarizes the effects of A. garckeana root-bark extract on the hematological parameters in P. berghei infected mice after the 4-day suppressive test. It shows that A. garckeana root-bark extract has a modulatory effect on hematological parameters in P. berghei infected mice, with the 300 mg/kg dose demonstrating more pronounced positive effects.
Table 2 summarizes the effects of A. garckeana root-bark extract on the hematological parameters in P. berghei infected mice after the 4-day suppressive test. It shows that A. garckeana root-bark extract has a modulatory effect on hematological parameters in P. berghei infected mice, with the 300 mg/kg dose demonstrating more pronounced positive effects.
Table 2. Effects of A. garckeana root-bark extract on the hematological level of P. berghei infected mice after the 4-day suppressive test
| Parameter | WBC (x109/L) | RBC (x1012/L) | PLT (x109/L) | PCV (%) | HGB (g/dL) | MCV (fl) | MHC (%) | LYM (x109/L) | NEU (x109/L) | BAS (%) | MCHC (%) | ||||||||
| Naïve | 11.70 ± 0.19 | 7.14 ± 0.15 | 1070.75 ± 24.36 | 38.28 ± 0.86 | 12.65 ± 0.38 | 54.73 ± 0.57 | 18.38 ± 0.59 | 87.63 ± 0.53 | 15.75 ± 0.63 | 1.03 ± 0.17 | 31.98 ± 0.89 | ||||||||
| Negative Control | 9.15 ± 0.22d | 3.97 ± 0.49d | 590.50 ± 24.55d | 21.83 ± 1.13d | 7.40 ± 0.69d | 51.00 ± 0.96 | 17.65 ± 0.64 | 80.48 ± 1.86d | 6.95 ± 0.62d | 0.48 ± 0.15 | 39.60 ± 1.66b | ||||||||
| Artemether | 16.93 ± 0.39ab | 7.24 ± 0.38a | 1090.75 ± 23.73a | 33.85 ± 0.29ad | 11.15 ± 1.22a | 54.30 ± 1.04 | 17.28 ± 0.49 | 79.10 ± 0.47d | 11.83 ± 0.89ad | 1.45 ± 0.30a | 31.83 ± 0.74c | ||||||||
| AZRE 100 mg/kg | 9.70 ± 0.68df | 3.88 ± 0.24df | 823.75 ± 36.46adf | 22.05 ± 0.70df | 7.33 ± 0.50df | 50.75 ± 0.77 | 19.20 ± 0.28e | 71.23 ± 1.55cdf | 8.68 ± 0.29df | 2.83 ± 0.27abe | 33.98 ± 0.36c | ||||||||
| AZRE 200 mg/kg | 13.90 ± 0.08abf | 5.34 ± 0.25adf | 928.50 ± 15.92adf | 29.28 ± 1.62adf | 10.08 ± 0.24ad | 53.13 ± 1.32 | 18.00 ± 0.83 | 72.13 ± 1.54cdf | 11.58 ± 0.77ad | 2.38 ± 0.19abe | 33.93 ± 0.31c | ||||||||
| AZRE 300 mg/kg | 17.08 ± 1.33ab | 6.82 ± 0.13a | 1037.50 ± 30.31a | 34.08 ± 1.25ad | 10.53 ± 0.40ad | 57.98 ± 3.36a | 18.08 ± 0.11 | 79.85 ± 1.23d | 13.53 ± 0.80ad | 2.1 ± 0.14abe | 31.03 ± 0.23c | ||||||||
aP < 0.05 higher compared to negative control; bP < 0.05 higher compared to normal control; cP < 0.05 lower compared to negative control; dP < 0.05 lower compared to normal control; eP < 0.05 higher compared to Artemether; fP < 0.05 lower compared to Artemether. RBC: Red blood cells, WBC: White blood cells, PLT: Platelets, PCV: Packed cell volume, HGB: Hemoglobin MCV: Mean corpuscular volume, MHC: Mean hemoglobin concentration, LYM: Lymphocytes, NEU: Neutrophils, BAS: Basophils, MCHC: Mean corpuscular hemoglobin concentration; AZRE: Azanza garckeana root-bark extract.
Discussion
Phytochemical Composition of the Root-bark Extract of Azanza garckeana:
The phytochemical screening of the aqueous extract of A. garckeana root bark revealed the presence of various bioactive compounds, aligning with previous studies on different parts of the plant (8,9,10). The identified phytochemicals, including saponins, tannins, flavonoids, alkaloids, glycosides, phenols, and steroids, provide a foundation for understanding the potential therapeutic effects of the root-bark extract against P. berghei in mice.
Parasitaemia and Chemosuppression Level of Treated P. berghei Infected Mice:
The 4-day suppressive test results on the effects of A. garckeana root-bark extract on the parasitaemia level of P. berghei-infected mice revealed promising outcomes, suggesting its significant antiplasmodial properties as a potential therapeutic agent for malaria treatment. In comparison to the negative control, which exhibited a steady increase in parasitaemia levels, and the artemether group, where parasitaemia levels decreased, the A. garckeana root-bark extract demonstrated a rapid and substantial significant reduction in parasitaemia levels but not surpassing the efficacy of artemether. The dose-dependent nature of the antiplasmodial activity was evident, with the 300 mg/kg dose exhibiting the most significant decrease in parasitaemia levels, aligning with previous studies highlighting the dose-dependent antiplasmodial effects of A. garckeana (20). These findings underscore the potential of A garckeana as a promising and dose-responsive alternative therapy for malaria (20).
The results of the 4-day suppressive test evaluating the effects of A. garckeana root-bark extract on the chemosuppression level in P. berghei-infected mice revealed a dose-dependent response. The negative control exhibited no chemosuppression, serving as a baseline for uncontrolled parasitemia growth. In comparison, the standard antimalarial drug, artemether, demonstrated a substantial increase in chemosuppression over the treatment period, reaching an impressive 96.09% on day 4. Notably, the A. garckeana root-bark extract groups at various concentrations (100 mg/kg, 200 mg/kg, and 300 mg/kg) exhibited dose-dependent chemosuppressive effects. The highest dose, 300 mg/kg, demonstrated a significant chemosuppression level of 88.17% on day 4, approaching the efficacy of artemether. These findings show the ability of A. garckeana to impede the development of P. berghei, indicating its potential utility as an antimalarial agent. This is consistent with the findings of a study on the antimalarial activity of Zingiber Officinale and Echinops Kebericho (21). Similar results were obtained by another study when using Salvadora persica root extract and Balanites rotundifolia leaf extract to prevent P. berghei growth (22).
The aqueous root-bark extract of A. garckeana demonstrated appreciable and dose-dependent chemosuppressive effects against P. berghei infection in mice. The identified phytochemicals, including flavonoids, saponins and alkaloids, likely mediate antiparasitic effects through well-established mechanisms of cell membrane disruption, protein binding, and heme polymerization to exert parasite clearance (23, 24). These multimodal actions help circumvent current drug resistance issues. About 88% suppression at 300 mg/kg is therapeutically relevant, considering prior studies demonstrating >60% efficacy as a cut-off for antimalarial potential (24).
The observed reduction in malaria parasite growth by the A. garckeana root-bark extract may occur in different ways that we are not fully aware of. It could enhance the immune system indirectly or block certain pathways in the body. The plant contains phytosteroids, phenolic compounds, and flavonoids, which are known for their potential to boost the immune system, reduce inflammation, and act as antioxidants. Additionally, these plant components might interact with the known factors involved in the development and life cycle of the malaria parasite, using a similar or unique method (26).
In this study, it was found that a high dose of the plant extract was effective against the parasite. By nanoformulating the extract, the same therapeutic outcomes may be achieved with lower doses, minimizing toxicity and improving patient compliance. Nanoformulation involves encapsulating active compounds into nanoparticles to enhance their effectiveness and reduce dosage requirements (27). This enhances the extract's solubility, stability, and targeted delivery, leading to better bioavailability and therapeutic outcomes. Additionally, it allows for controlled release kinetics, optimizing treatment strategies (27). Overall, nanoformulation of medicinal plant extracts shows promise for improving antimalarial therapies and herbal medicine in general (28).
Hematological Level:
The hematological analysis following the 4-day suppressive test with A. garckeana root-bark extract on P. berghei-infected mice reveals notable impacts on various blood parameters. In comparison to the naive (non-infected) group, the negative control group, representing untreated infected mice, exhibited significant reductions in key parameters, including white blood cells (WBC), red blood cells (RBC), platelets (PLT), packed cell volume (PCV), and hemoglobin (HGB). These reductions align with the expected effects of P. berghei infection on blood components. Artemether, the standard antimalarial drug, showed some restoration of these parameters, indicating its efficacy in mitigating malaria-induced hematological alterations. Interestingly, A. garckeana at different doses demonstrated varying effects on the hematological parameters which concise other findings on Ocimum gratissimum (29) and Jatropha tanjorensis leaf (30). The 300 mg/kg dose of A. garckeana showed an increase in WBC, RBC, PLT, PCV, and HGB compared to the negative control, suggesting a potential protective effect against malaria-induced hematological changes. However, some parameters, such as mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and lymphocytes (LYM), showed mixed responses across A. garckeana groups. These findings suggest that A. garckeana root-bark extract may have a modulatory effect on hematological parameters in P. berghei-infected mice, with the 300 mg/kg dose demonstrating more pronounced positive effects.
The study revealed that administering an aqueous root-bark extract of A. garckeana to mice infected with P. berghei resulted in a significant increase in white blood cells (WBC) and neutrophil (NEU) counts when compared to the uninfected control group. With 100 and 200 mg/kg A. garckeana compared to naïve mice, basophil percentages were similarly increased.
White blood cells play an important role in the immune response against malarial infection. Specifically, neutrophils are involved in the initial innate response and help control parasite multiplication through phagocytosis and cytokine release (31). The observed increase in WBCs and neutrophils suggests that A. garckeana extract may stimulate immune cell production and activity, enhancing the clearance of parasitized erythrocytes. This immune-boosting effect could contribute to the antimalarial efficacy noted in prior in vivo studies testing extracts of this plant (26).
Elevations in circulating basophils were also seen with 100 and 200 mg/kg A. garckeana extract treatment. Though the function of basophils in malaria is less clear, some evidence suggests they may play a regulatory role through interactions with T-cells and the release of IL-4 (32). The higher percentages could reflect increased activation and recruitment related to the extract's influence on immune processes during infection.
No significant alterations were detected between groups for other hematological indices like red blood cells, hemoglobin, platelets, or red cell volume and morphology. The lack of major changes indicates the extract does not cause haematoxicity and is well-tolerated at the tested doses. Artemether similarly did no effect on these measures at its curative concentration.
Conclusion
The medicinal plant A. garckeana's aqueous root-bark extract showed strong antiplasmodial action against P. berghei infection in mice. Significant chemosuppression was attained, and hematological parameters were modulated by it. The findings confirm the ethnomedical significance of A. garckeana and encourage more research into the phytochemical components of the plant to develop antimalarial medications. However, limited access to advanced analytical tools like gas chromatography and mass spectrometry hindered detailed characterization of the plant's active compounds. Also, the majority of previous studies that sought to confirm the antimalarial properties of plants employed methodologies akin to ours. Despite this, the plant's antimalarial potential warrants further exploration, particularly in isolating and characterizing its active compounds. Additionally, exploring nanoformulation of the plant extract could offer promising avenues to enhance its therapeutic benefits and reduce dosage requirements.
Acknowledgments
The authors sincerely appreciate Biochemistry Department of Modibbo Adama University, Yola for allowing them to use the departmental laboratory and other necessary facilities for the success of this study.
Ethical Statement
The Animal Ethics Committee at the Department of Animal Science, Faculty of Agricultural Science, Modibbo Adama University, Yola, gave the clearance and authorization for the use of animals in this study (MAU/FAS/AEC/AS/2023/036).
Data availability
The supporting data of this study is always obtainable on request from the corresponding author.
Authors’ contributions
Author MSJ designed the study, conducted the statistical analysis and wrote the first draft of the manuscript; author YU performed the experiments; author AUW managed the analyses of the study and literature searches. All authors read and approved the final manuscript.
Funding/Support
No funding support received for this work. The work is fully funded by the authors.
Conflict of interest
The authors have no conflict of interest in this study.
Type of Study: orginal article |
Subject:
Parasitology
References
1. Oladipo HJ, Tajudeen YA, Oladunjoye IO, Yusuff SI, Yusuf RO, Oluwaseyi EM, et al. Increasing challenges of malaria control in sub-Saharan Africa: Priorities for public health research and policymakers. Ann Med Surg 2022;81. http://dx.doi.org/10.1016/j.amsu.2022.104366 [DOI:10.1016/j.amsu.2022.104366]
2. Venkatesan P. The 2023 WHO World malaria report. Lancet Microbe 2024;5(3):e214. http://dx.doi.org/10.1016/s2666-5247(24)00016-8 [DOI:10.1016/S2666-5247(24)00016-8] [PMID]
3. Nyandwaro K, Oyweri J, Kimani F, Mbugua A. Evaluating Antiplasmodial and Antimalarial Activities of Soybean (Glycine max) Seed Extracts on P. falciparum Parasite Cultures and Plasmodium berghei-Infected Mice. J. Pathog 2020:1-8. http://dx.doi.org/10.1155/2020/7605730 [DOI:10.1155/2020/7605730] [PMID] []
4. van der Pluijm RW, Amaratunga C, Dhorda M, Dondorp AM. Triple Artemisinin-Based Combination Therapies for Malaria - A New Paradigm? Trends Parasitol 2021;37(1):15-24. http://dx.doi.org/10.1016/j.pt.2020.09.011 [DOI:10.1016/j.pt.2020.09.011] [PMID]
5. Obakiro SB, Kiprop A, Kowino I, Kigondu E, Odero MP, Omara T, et al. Ethnobotany, ethnopharmacology, and phytochemistry of traditional medicinal plants used in the management of symptoms of tuberculosis in East Africa: a systematic review. Trop Med Health 2020;48(1). http://dx.doi.org/10.1186/s41182-020-00256-1 [DOI:10.1186/s41182-020-00256-1] [PMID] []
6. Maroyi A. Azanza garckeana Fruit Tree: Phytochemistry, Pharmacology, Nutritional and Primary Healthcare Applications as Herbal Medicine: A Review. Res J Med Plant 2017;11(4):115-23. http://dx.doi.org/10.3923/rjmp.2017.115.123 [DOI:10.3923/rjmp.2017.115.123]
7. Cowman AF, Healer J, Marapana D, Marsh K. Malaria: Biology and Disease. Cell. 2016;167(3):610-24. http://dx.doi.org/10.1016/j.cell.2016.07.055 [DOI:10.1016/j.cell.2016.07.055] [PMID]
8. Yusuf AA, Lawal B, Sani S, Garba R, Mohammed BA, Oshevire DB, et al. Pharmacological activities of Azanza garckeana (Goron Tula) grown in Nigeria. Clin. Phytoscience. 2020;6(1). http://dx.doi.org/10.1186/s40816-020-00173-0 [DOI:10.1186/s40816-020-00173-0]
9. Momodu IB, Agoreyo BO, Okungbowa ES, Igiebor SE, Igbo IOL. Phytochemical screening and proximate composition of aqueous-methanol pulp extract of Azanza garckeana (Goron Tula). Dutse J Pure Appl Sci 2022;7(4a):194-200. http://dx.doi.org/10.4314/dujopas.v7i4a.20 [DOI:10.4314/dujopas.v7i4a.20]
10. Momodu IB, Agoreyo BO, Okungbowa ES, Igiebor SE, Igbo IOL. Phytochemical screening and proximate composition of aqueous-methanol pulp extract of Azanza garckeana (Goron Tula). Dutse J Pure Appl Sci 2022;7(4a):194-200. http://dx.doi.org/10.4314/dujopas.v7i4a.20 [DOI:10.4314/dujopas.v7i4a.20]
11. Mohammadi S, Jafari B, Asgharian P, Martorell M, Sharifi‐Rad J. Medicinal plants used in the treatment of Malaria: A key emphasis to Artemisia, Cinchona, Cryptolepis, and Tabebuia genera. Phytother Res 2020;34(7):1556-69. http://dx.doi.org/10.1002/ptr.6628 [DOI:10.1002/ptr.6628] [PMID]
12. Gujjari L, Kalani H, Pindiprolu SK, Arakareddy BP, Yadagiri G. Current challenges and nanotechnology-based pharmaceutical strategies for the treatment and control of malaria. Parasite Epidemiol. Control 2022;17:e00244. http://dx.doi.org/10.1016/j.parepi.2022.e00244 [DOI:10.1016/j.parepi.2022.e00244] [PMID] []
13. Woodrow CJ, White NJ. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol Rev 2016;41(1):34-48. http://dx.doi.org/10.1093/femsre/fuw037 [DOI:10.1093/femsre/fuw037] [PMID] []
14. Hamilton A, Haghpanah F, Hasso-Agopsowicz M, Frost I, Lin G, Schueller E, et al. Malaria Vaccine Impact on Drug-Susceptible and Resistant Cases and Deaths: A Modeling Study. SSRN Electron J 2022. http://dx.doi.org/10.2139/ssrn.4231231 [DOI:10.2139/ssrn.4231231]
15. Asakitikpi A. Healthcare Coverage and Affordability in Nigeria: An Alternative Model to Equitable Healthcare Delivery. Universal Healthcare [Working Title]. 2019; http://dx.doi.org/10.5772/intechopen.85978 [DOI:10.5772/intechopen.85978]
16. Benmerache A, Berrehal D, Khalfallah A, Kabouche A, Semra Z, Kabouche Z. Total phenolic quantification, antioxidant, antibacterial activities and flavonoids of Algerian Calotropis procera (Asclepiadaceae). Der Pharm Lett 2013;5(4):204-7. [URL]
17. Evans WC. Trease and Evans' Pharmacognosy. Elsevier Health Sciences; 2009. http://books.google.ie/books?id=l7pkTFyY428C&printsec=frontcover&dq=Trease+GE,+Evans+WC.+Textbook+of+pharmacognosy.+13th+ed.+London,+UK%3B+Toronto,+Canada%3B+Tokyo,+Japan:+Bailiere+Tindall%3B+1989.+pp.+200%E2%80%931.&hl=&cd=2&source=gbs_api [GOOGLE BOOK]
18. Gitua JN, Muchiri DR, and Nguyen XA. In vivo antimalarial activity of Ajuga remota water extracts against Plasmodium berghei in mice. Southeast Asian J Trop Med Public Health 2012;43(3):545-8. [PMID]
19. Dikasso D, Makonnen E, Debella A, Abebe D, Urga K, Makonnen W, et al. In vivo anti-malarial activity of hydroalcoholic extracts from Asparagus africanus Lam. in mice infected with Plasmodium berghei. Ethiop J Health Dev 2007;20(2). http://dx.doi.org/10.4314/ejhd.v20i2.10021 [DOI:10.4314/ejhd.v20i2.10021]
20. Connelly MPE, Fabiano E, Patel IH, Kinyanjui SM, Mberu EK, Watkins WM. Antimalarial activity in crude extracts of Malawian medicinal plants. Ann Trop Med Parasitol 1996;90(6):597-602. http://dx.doi.org/10.1080/00034983.1996.11813089 [DOI:10.1080/00034983.1996.11813089] [PMID]
21. Biruksew A, Zeynudin A, Alemu Y, Golassa L, Yohannes M, Debella A, et al. Zingiber Officinale Roscoe and Echinops Kebericho Mesfin Showed Antiplasmodial Activities against Plasmodium Berghei in a Dosedependent Manner in Ethiopia. Ethiop J Health Sci 2018;28(5). http://dx.doi.org/10.4314/ejhs.v28i5.17 [DOI:10.4314/ejhs.v28i5.17] [PMID] []
22. Gebrehiwot S, Shumbahri M, Eyado A, Yohannes T. Phytochemical Screening and In Vivo Antimalarial Activity of Two Traditionally Used Medicinal Plants of Afar Region, Ethiopia, against Plasmodium berghei in Swiss Albino Mice. J Parasitol Res 2019:1-8. http://dx.doi.org/10.1155/2019/4519298 [DOI:10.1155/2019/4519298] [PMID] []
23. Bickii J, Tchouya G, Tchouankeu J, Tsamo E. Antimalarial activity in crude extracts of some Cameroonian medicinal plants. Afr J Tradit Complement Altern Med 2007;4(1). http://dx.doi.org/10.4314/ajtcam.v4i1.31200 [DOI:10.4314/ajtcam.v4i1.31200] [PMID] []
24. Lawal B, Sani S, Onikanni AS, Ibrahim YO, Agboola AR, Lukman HY, et al. Preclinical anti-inflammatory and antioxidant effects of Azanza garckeana in STZ-induced glycemic-impaired rats, and pharmacoinformatics of it major phytoconstituents. Biomed Pharmacother 2022; 152:113196. http://dx.doi.org/10.1016/j.biopha.2022.113196 [DOI:10.1016/j.biopha.2022.113196] [PMID]
25. Chaniad P, Techarang T, Phuwajaroanpong A, Plirat W, Viriyavejakul P, Septama AW, et al. Antimalarial efficacy and toxicological assessment of medicinal plant ingredients of Prabchompoothaweep remedy as a candidate for antimalarial drug development. BMC Complement Med Ther 2023; 23(1). http://dx.doi.org/10.1186/s12906-023-03835-x [DOI:10.1186/s12906-023-03835-x] [PMID] []
26. Habte G, Assefa S. In Vivo Antimalarial Activity of Crude Fruit Extract of Capsicum frutescens Var. Minima (Solanaceae) against Plasmodium berghei-Infected Mice. Biomed Res Int 2020;25:1-7. http://dx.doi.org/10.1155/2020/1320952 [DOI:10.1155/2020/1320952] [PMID] []
27. Han HS, Koo SY, Choi KY. Emerging nanoformulation strategies for phytocompounds and applications from drug delivery to phototherapy to imaging. Bioactive Materials 2022;14:182-205. http://dx.doi.org/10.1016/j.bioactmat.2021.11.027 [DOI:10.1016/j.bioactmat.2021.11.027] [PMID] []
28. Firooziyan S, Osanloo M, Basseri HR, Moosa-Kazemi SH, Mohammadzadeh Hajipirloo H, Amani A, et al. Nanoemulsion of Myrtus communis essential oil and evaluation of its larvicidal activity against Anopheles stephensi. Arabian J Chem 2022;15(9):104064. http://dx.doi.org/10.1016/j.arabjc.2022.104064 [DOI:10.1016/j.arabjc.2022.104064]
29. Ofem O, Ani E, Eno A. Effect of aqueous leaves extract of Ocimum gratissimum on hematological parameters in rats. Int J Appl Basic Medl Res 2012;2(1):38. http://dx.doi.org/10.4103/2229-516x.96807 [DOI:10.4103/2229-516X.96807] [PMID] []
30. Igbinaduwa P, Usifoh C, Ugwu C. Phytochemical analysis and toxicological evaluation of the methanolic extract of Jatropha tanjorensis leaf. J Pharm Bioresour 2012;8(2). http://dx.doi.org/10.4314/jpb.v8i2.4 [DOI:10.4314/jpb.v8i2.4]
31. Malech HL, DeLeo FR, Quinn MT. The Role of Neutrophils in the Immune System: An Overview. Neutrophil Methods Protocols 2014;3-10. http://dx.doi.org/10.1007/978-1-62703-845-4_1 [DOI:10.1007/978-1-62703-845-4_1] [PMID] []
32. Donnelly EL, Céspedes N, Hansten G, Wagers D, Briggs AM, Lowder C, et al. Basophil Depletion Alters Host Immunity, Intestinal Permeability, and Mammalian Host-to-Mosquito Transmission in Malaria. ImmunoHorizons 2022;6(8):581-99. http://dx.doi.org/10.4049/immunohorizons.2200055 [DOI:10.4049/immunohorizons.2200055] [PMID] []
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







 gmail.com
gmail.com