Volume 10, Issue 3 (7-2024)
Journal of Research in Applied and Basic Medical Sciences 2024, 10(3): 285-290 |
Back to browse issues page
Ethics code: 1346/IECBMHR/GMC/2024
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mehta A, Mungi V, Jain S. Prevalence and antimicrobial susceptibility profile of clinical isolates of Citrobacter spp. at a tertiary care hospital in Madhya Pradesh. Journal of Research in Applied and Basic Medical Sciences 2024; 10 (3) :285-290
URL: http://ijrabms.umsu.ac.ir/article-1-343-en.html
URL: http://ijrabms.umsu.ac.ir/article-1-343-en.html
Associate Professor, Department of Microbiology, Government Medical College, Datia, Madhya Pradesh, India , abhishekmehta623@gmail.com
Full-Text [PDF 329 kb]
(542 Downloads)
| Abstract (HTML) (1123 Views)
.jpg)
Table 1. Comparison of Antibiotic susceptibility pattern of Citrobacter species in various studies with our study (%)
Discussion
As evident from the results of our study, amongst all Citrobacter spp. isolates, C. koseri was predominantly isolated (52%) followed by C. freundii (46%) from urine and pus cultures respectively. Like our study, Kalpana S et al also reported a remarkable predominance of C.koseri to the extent of 68 % followed by C. freundii (32%) (3).
In another similar study conducted by Nayar R et al., wherein 526 Citrobacter spp. isolates were obtained from a total of 24,442 samples processed. A prevalence rate of 2.1% was reported, which is slightly lower than our study. In this study, C.koseri (42%) was identified as the commonest Citrobacter species isolated from clinical samples followed by C.freundii (19%). This finding is in concordance with our study though here the other Citrobacter spp. (27%) constituted a big chunk of total isolates (6).
A sample-wise analysis obtained from further studies as compared to ours showed that C. koseri and C. freundii were predominantly isolated from urine and pus samples as compared to other species of the genus. Other samples like blood, sputum, and other fluids yielded a very low incidence of the bacteria, however, the incidence of the bacterium remains ubiquitous and is not limited to a particular sample. The antimicrobial susceptibility pattern of the study isolates of Citrobacter spp., 50% of isolates showed high sensitivity towards carbapenems (86%), followed by piperacillin-tazobactam (68%), fluoroquinolones (62%), cefazolin (60%) and gentamicin (54%). However, more than 50% of the isolates exhibited low susceptibility towards amoxicillin-clavulanate, ampicillin-sulbactam, third-generation cephalosporins (3GC), amikacin, and nitrofurantoin. These findings were in concordance with the studies conducted by Dhanya A et al.(7), Dhason MT et al.(8), Ranjan KP et al.(9), Basavaraj C et al.(10), Priyadarshini et al.(11) who also reported that the susceptibility towards carbapenems was highest, which is >80%, but showed mixed results with antibiotics like 1st generation cephalosporins, fluoroquinolones, aminoglycosides, nitrofurantoin, and piperacillin-tazobactam, with a susceptibility ranging from 50% - 70%, but an absolute resistance pattern was observed towards amoxicillin-clavulanate, 3rd generation cephalosporins. As we observed more, studies conducted by Rizvi M et al.(12) and Kumar S. et al.(13) determined that carbapenems, nitrofurantoin, aminoglycosides, and piperacillin-tazobactam were primarily used as empirical therapy for the treatment of the disease caused by Citrobacter spp. and drugs like amoxicillin-clavulanate, cephalosporins, cotrimoxazole, and fluoroquinolones have been observed to be of low advantage. The comparative susceptibility for various antibiotics in other studies and our study has been discussed in Table 1. This property can be attributed to the production of Klebsiella pneumoniae carbapenemase-2 (KPC-2) and decreased expression of porinswhich can effectively hydrolyze and render many antibiotics ineffective like penicillin and its derivatives, although high sensitivity towards carbapenems, aminoglycosides, and piperacillin-tazobactam indicate that the incidence is still low but resistance is gradually on the rise, which may be due to the non-judicial use of antibiotics and lack of antimicrobial stewardship laws in a developing country like India.
A study conducted by Praharaj et al.(14) also used sophisticated molecular detection modalities like Vitek-2, multi-locus sequence typing, and polymerase chain reaction along with phenotypic detection of beta-lactamases, successfully testing 221 isolates comprising of 130 C. freundii isolates and 91 C. koseri isolates, in which, 107 C. freundii isolates (82.3%) and 72 C. koseri isolates (79.1%) were found to have carbapenem resistance with MIC values for imipenem, meropenem, and ertapenem ranging from 8 to 32 µg/ml as per CLSI breakpoints with the presence of blaNDM-1and blaVIMgenes.
The acquisition of these genes by mechanisms like horizontal gene transfer and mutations which enable the bacteria to avoid or escape the action of certain antibiotics has become so common over the years that the Clinical Laboratory Standards Institute (CLSI) has directed medical practitioners not to use certain antibiotics as Citrobacter spp. have been reported to be intrinsically resistant to them. Intrinsic resistance of C. freundii towards ampicillin, amoxicillin-clavulanate, ampicillin-sulbactam, 1st generation cephalosporins like cefazolin and cephalothin, cephamycins like cefoxitin and cefotetan and that of C. koseri towards ampicillin and ticarcillin has been reported and guidelines have been issued that despite of moderate in vitro susceptibility of these antibiotics, use of these antibiotics in clinical practice may not be necessary because of unsatisfactory in vivo performance and only 1-3% of isolates may still be susceptible (15), which is in concordance with our study except for cefazolin and ampicillin-sulbactam which showed a susceptibility of 32% and 60% respectively. This discordance may be due to the method used and the sample size in our study.
So, as we compare the antibiotic resistance in Table 1, it is evident that the highest level of sensitivity was observed with carbapenems in all the studies including ours which establish the fact that carbapenems are the treatment of choice and moderate sensitivity was shown for piperacillin-tazobactam and aminoglycosides. Our study also confirmed the intrinsic resistance towards ampicillin, ampicillin-sulbactam, and amoxicillin-clavulanic acid which is in concordance with CLSI guidelines.
Limitations
Conclusion
The findings of the study indicate a change in susceptibility trends of emerging pathogens like Citrobacter spp. exhibiting resistance to routinely prescribed antimicrobials. Increasing antimicrobial resistance amongst such nosocomial pathogens is posing a serious challenge in the management of such infectionsstressing the need for an appropriate action plan involving antimicrobial stewardship, and strict implementation of infection control practices.
Appropriate Surveillance strategy for regular continuous monitoring of antimicrobial susceptibility patterns with the formulation of antibiotic policy at the institutional or regional level is an important prerequisite to rationalize antimicrobial treatment protocols to curb the menace of rapidly emerging drug resistance amongst such pathogens.
Acknowledgements
Nil
Ethical statement
This study was conducted in accordance with the ethical guidelines approved by the Institutional Ethics Committee (IEC) with the code 1346/IECBMHR/GMC/2024.
Author Contributions
All the authors have contributed significantly to the conception; acquisition, analysis, interpretation of data; drafting and reviewing of the manuscript. All the authors gave approval to the final version of the manuscript and agreed to be accountable for all aspects of the study.
Conflict of interest
There are no conflicts of interest.
Funding
Nil
Full-Text: (186 Views)
Introduction
Citrobacter, a member of the Enterobacteriaceae family is known to be a normal flora of the intestine and an opportunistic pathogen often commonly found in the intestinal tract of humans and animals, soil, and sewage. The genus Citrobacter and the species C.freundii were designated in 1932 by Werkman and Gillen. In 1970, Frederiksen described a new species that he named C.koseri.In 1993, Brenner and colleagues, using DNA relatedness studies, showed that organisms identified as C. freundii consisted of a heterogeneous group representing several genetic species and were included in the C. freundii complex comprising of C. freundii, C.youngae, C.braakii, C.werkmanii, C.sedlakii, C.rodentium, C.gillenii and C.murliniae (1).
It is now increasingly found to cause a variety of infections in community as well as hospital settings. Earlier this pathogen was known to exhibit low virulence but is now found to cause multi-drug resistant infections with high morbidity and mortality (2).
This is attributed to ineffective empirical therapy, and irrational prescription practices instead of culture-guided therapy which, over the years, has led to the production of enzymes known as beta-lactamases, which can hydrolyze beta-lactam antibiotics and their derivatives like cephalosporins and low incidence of resistance towards carbapenems, but increasing as time passes. C.koseri and C.freundii have been reported as the most common isolates among other species in this genus. These species in this genus are known to cause a wide range of infections including brain abscesses, gastroenteritis, pneumonia, endocarditis, wound infections, septicemia, meningitis, and urinary tract infections (UTIs), particularly in neonates and immunocompromised patients (3).
In this part of the country, very few studies have been conducted to determine the prevalence, virulence, pathogenesis, and antimicrobial resistance pattern of the infections caused by Citrobacter spp. Due to the paucity of such data in this region which is imperative in formulating the strategy for the management of such infections, a cross-sectional study was planned and undertaken at a tertiary care teaching hospital in Central India retrospectively to determine the prevalence of the infections caused by Citrobacter spp. and their antimicrobial sensitivity pattern.
Material & Methods
Study design: A Laboratory records based Cross-sectional study done retrospectively
Sampling & sample size: Laboratory data about 50 Citrobacter spp. isolates (antimicrobial susceptibility profile) obtained from 1628 clinical samples processed in the bacteriology lab. of Dept. of Microbiology, Government Medical College, Datia (M.P.)-India.
Duration: 18 months (July 2022 to Dec.2023)
Laboratory data of the isolates under study was retrieved and analyzed after obtaining permission from concerned authorities. The confidentiality regarding the identity and personal details of study participants was maintained throughout the study.
The isolates were identified using Standard microbiological techniques and antimicrobial sensitivity tests done by the Kirby Bauer disk diffusion method and interpreted as per CLSI 2022 guidelines.Data collection and analysis were done using MS Office Excel 2021. Statistical analysis was done using descriptive statistics, presented as frequencies and percentages in tables and graphs. The Unpaired t-test (student t-test) was applied to determine the levels of significance (P <0.05 was considered significant) (1-5).
Results
Out of the 1628 samples processed during the study period, 770 samples were found to yield significant bacterial growth. Out of the 770 clinical isolates, 50 samples were found to be positive for Citrobacter spp. (6.4%). Isolation rate for Citrobacter spp. was reported to be 3.1 %.
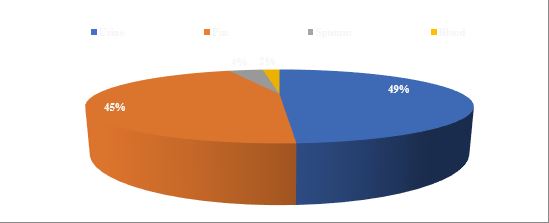
The majority of isolates were obtained from Urine (49%) and pus samples (45%) with C. koseri being dominant in urine and C. freundii in pus. Sputum (4%) and Blood cultures (2%) yielded very few isolates (Figure 1).
Amongst 50 isolates, 26 (52%) were C.koseri, 22 (46%) were C.freundii and 2 (4%) were other species.
Citrobacter spp. isolates were found to exhibit the highest sensitivity towards Carbapenems followed by Piperacillin-tazobactam, Cefazolin, Ciprofloxacin, and Aminoglycosides. But were found to be poorly susceptible to Amoxycillin clavulanate, 3rd and 4th generation cephalosporins. All urinary isolates were susceptible to Nitrofurantoin. Multidrug resistance was seen in eight isolates (16%), mostly derived from pus samples (Figure 2).
Citrobacter, a member of the Enterobacteriaceae family is known to be a normal flora of the intestine and an opportunistic pathogen often commonly found in the intestinal tract of humans and animals, soil, and sewage. The genus Citrobacter and the species C.freundii were designated in 1932 by Werkman and Gillen. In 1970, Frederiksen described a new species that he named C.koseri.In 1993, Brenner and colleagues, using DNA relatedness studies, showed that organisms identified as C. freundii consisted of a heterogeneous group representing several genetic species and were included in the C. freundii complex comprising of C. freundii, C.youngae, C.braakii, C.werkmanii, C.sedlakii, C.rodentium, C.gillenii and C.murliniae (1).
It is now increasingly found to cause a variety of infections in community as well as hospital settings. Earlier this pathogen was known to exhibit low virulence but is now found to cause multi-drug resistant infections with high morbidity and mortality (2).
This is attributed to ineffective empirical therapy, and irrational prescription practices instead of culture-guided therapy which, over the years, has led to the production of enzymes known as beta-lactamases, which can hydrolyze beta-lactam antibiotics and their derivatives like cephalosporins and low incidence of resistance towards carbapenems, but increasing as time passes. C.koseri and C.freundii have been reported as the most common isolates among other species in this genus. These species in this genus are known to cause a wide range of infections including brain abscesses, gastroenteritis, pneumonia, endocarditis, wound infections, septicemia, meningitis, and urinary tract infections (UTIs), particularly in neonates and immunocompromised patients (3).
In this part of the country, very few studies have been conducted to determine the prevalence, virulence, pathogenesis, and antimicrobial resistance pattern of the infections caused by Citrobacter spp. Due to the paucity of such data in this region which is imperative in formulating the strategy for the management of such infections, a cross-sectional study was planned and undertaken at a tertiary care teaching hospital in Central India retrospectively to determine the prevalence of the infections caused by Citrobacter spp. and their antimicrobial sensitivity pattern.
Material & Methods
Study design: A Laboratory records based Cross-sectional study done retrospectively
Sampling & sample size: Laboratory data about 50 Citrobacter spp. isolates (antimicrobial susceptibility profile) obtained from 1628 clinical samples processed in the bacteriology lab. of Dept. of Microbiology, Government Medical College, Datia (M.P.)-India.
Duration: 18 months (July 2022 to Dec.2023)
Laboratory data of the isolates under study was retrieved and analyzed after obtaining permission from concerned authorities. The confidentiality regarding the identity and personal details of study participants was maintained throughout the study.
The isolates were identified using Standard microbiological techniques and antimicrobial sensitivity tests done by the Kirby Bauer disk diffusion method and interpreted as per CLSI 2022 guidelines.Data collection and analysis were done using MS Office Excel 2021. Statistical analysis was done using descriptive statistics, presented as frequencies and percentages in tables and graphs. The Unpaired t-test (student t-test) was applied to determine the levels of significance (P <0.05 was considered significant) (1-5).
Results
Out of the 1628 samples processed during the study period, 770 samples were found to yield significant bacterial growth. Out of the 770 clinical isolates, 50 samples were found to be positive for Citrobacter spp. (6.4%). Isolation rate for Citrobacter spp. was reported to be 3.1 %.
The majority of isolates were obtained from Urine (49%) and pus samples (45%) with C. koseri being dominant in urine and C. freundii in pus. Sputum (4%) and Blood cultures (2%) yielded very few isolates (Figure 1).
Amongst 50 isolates, 26 (52%) were C.koseri, 22 (46%) were C.freundii and 2 (4%) were other species.
Citrobacter spp. isolates were found to exhibit the highest sensitivity towards Carbapenems followed by Piperacillin-tazobactam, Cefazolin, Ciprofloxacin, and Aminoglycosides. But were found to be poorly susceptible to Amoxycillin clavulanate, 3rd and 4th generation cephalosporins. All urinary isolates were susceptible to Nitrofurantoin. Multidrug resistance was seen in eight isolates (16%), mostly derived from pus samples (Figure 2).

Fig. 1. Sample-wise distribution of the Citrobacter spp. isolates
.jpg)
Fig. 2. Antimicrobial Sensitivity pattern of Citrobacter spp. Isolates
Table 1. Comparison of Antibiotic susceptibility pattern of Citrobacter species in various studies with our study (%)
| Antibiotic Study |
AMC | AK | GEN | PIT | AMS | CAZ | CTR | CTX | CPM | CZ | MRP | IPM | CIP | NX | NIT |
| Dhanya A et al | - | 52 | 47.5 | 80 | 15 | 51 | 51 | 51 | - | - | 90 | 90 | 30 | 30 | - |
| Dhason MT et al | 40 | - | 75 | 86 | - | - | 59 | 58 | - | - | - | 90 | 70 | - | 33 |
| Basavaraj C et al | 15 | 60 | 19 | - | - | - | - | 87 | - | - | - | 90 | 40 | 30 | 60 |
| Priyadarshini et al | 39 | 40 | 31 | 50 | - | 31 | - | 33 | - | - | 98 | 98 | 30 | 30 | 63 |
| Rizvi M et al | - | 85.2 | 77.4 | 23.1 | - | - | 50.9 | 43.3 | - | - | - | 100 | 56.2 | - | 66.1 |
| Kumar S et al | - | 38 | 50 | - | - | - | - | - | - | - | - | - | - | - | - |
| Sofie A et al | - | 98 | 100 | 80 | - | - | - | - | 100 | - | - | 98 | - | - | 90 |
| Our Study | 8 | 40 | 54 | 68 | 32 | 40 | 14 | 14 | 12 | 60 | 84 | 86 | 62 | 54 | 25 |
As evident from the results of our study, amongst all Citrobacter spp. isolates, C. koseri was predominantly isolated (52%) followed by C. freundii (46%) from urine and pus cultures respectively. Like our study, Kalpana S et al also reported a remarkable predominance of C.koseri to the extent of 68 % followed by C. freundii (32%) (3).
In another similar study conducted by Nayar R et al., wherein 526 Citrobacter spp. isolates were obtained from a total of 24,442 samples processed. A prevalence rate of 2.1% was reported, which is slightly lower than our study. In this study, C.koseri (42%) was identified as the commonest Citrobacter species isolated from clinical samples followed by C.freundii (19%). This finding is in concordance with our study though here the other Citrobacter spp. (27%) constituted a big chunk of total isolates (6).
A sample-wise analysis obtained from further studies as compared to ours showed that C. koseri and C. freundii were predominantly isolated from urine and pus samples as compared to other species of the genus. Other samples like blood, sputum, and other fluids yielded a very low incidence of the bacteria, however, the incidence of the bacterium remains ubiquitous and is not limited to a particular sample. The antimicrobial susceptibility pattern of the study isolates of Citrobacter spp., 50% of isolates showed high sensitivity towards carbapenems (86%), followed by piperacillin-tazobactam (68%), fluoroquinolones (62%), cefazolin (60%) and gentamicin (54%). However, more than 50% of the isolates exhibited low susceptibility towards amoxicillin-clavulanate, ampicillin-sulbactam, third-generation cephalosporins (3GC), amikacin, and nitrofurantoin. These findings were in concordance with the studies conducted by Dhanya A et al.(7), Dhason MT et al.(8), Ranjan KP et al.(9), Basavaraj C et al.(10), Priyadarshini et al.(11) who also reported that the susceptibility towards carbapenems was highest, which is >80%, but showed mixed results with antibiotics like 1st generation cephalosporins, fluoroquinolones, aminoglycosides, nitrofurantoin, and piperacillin-tazobactam, with a susceptibility ranging from 50% - 70%, but an absolute resistance pattern was observed towards amoxicillin-clavulanate, 3rd generation cephalosporins. As we observed more, studies conducted by Rizvi M et al.(12) and Kumar S. et al.(13) determined that carbapenems, nitrofurantoin, aminoglycosides, and piperacillin-tazobactam were primarily used as empirical therapy for the treatment of the disease caused by Citrobacter spp. and drugs like amoxicillin-clavulanate, cephalosporins, cotrimoxazole, and fluoroquinolones have been observed to be of low advantage. The comparative susceptibility for various antibiotics in other studies and our study has been discussed in Table 1. This property can be attributed to the production of Klebsiella pneumoniae carbapenemase-2 (KPC-2) and decreased expression of porinswhich can effectively hydrolyze and render many antibiotics ineffective like penicillin and its derivatives, although high sensitivity towards carbapenems, aminoglycosides, and piperacillin-tazobactam indicate that the incidence is still low but resistance is gradually on the rise, which may be due to the non-judicial use of antibiotics and lack of antimicrobial stewardship laws in a developing country like India.
A study conducted by Praharaj et al.(14) also used sophisticated molecular detection modalities like Vitek-2, multi-locus sequence typing, and polymerase chain reaction along with phenotypic detection of beta-lactamases, successfully testing 221 isolates comprising of 130 C. freundii isolates and 91 C. koseri isolates, in which, 107 C. freundii isolates (82.3%) and 72 C. koseri isolates (79.1%) were found to have carbapenem resistance with MIC values for imipenem, meropenem, and ertapenem ranging from 8 to 32 µg/ml as per CLSI breakpoints with the presence of blaNDM-1and blaVIMgenes.
The acquisition of these genes by mechanisms like horizontal gene transfer and mutations which enable the bacteria to avoid or escape the action of certain antibiotics has become so common over the years that the Clinical Laboratory Standards Institute (CLSI) has directed medical practitioners not to use certain antibiotics as Citrobacter spp. have been reported to be intrinsically resistant to them. Intrinsic resistance of C. freundii towards ampicillin, amoxicillin-clavulanate, ampicillin-sulbactam, 1st generation cephalosporins like cefazolin and cephalothin, cephamycins like cefoxitin and cefotetan and that of C. koseri towards ampicillin and ticarcillin has been reported and guidelines have been issued that despite of moderate in vitro susceptibility of these antibiotics, use of these antibiotics in clinical practice may not be necessary because of unsatisfactory in vivo performance and only 1-3% of isolates may still be susceptible (15), which is in concordance with our study except for cefazolin and ampicillin-sulbactam which showed a susceptibility of 32% and 60% respectively. This discordance may be due to the method used and the sample size in our study.
So, as we compare the antibiotic resistance in Table 1, it is evident that the highest level of sensitivity was observed with carbapenems in all the studies including ours which establish the fact that carbapenems are the treatment of choice and moderate sensitivity was shown for piperacillin-tazobactam and aminoglycosides. Our study also confirmed the intrinsic resistance towards ampicillin, ampicillin-sulbactam, and amoxicillin-clavulanic acid which is in concordance with CLSI guidelines.
Limitations
- Due to the lack of adequate funding and resources, the detection of beta-lactamases was not performed and the study was limited to the reporting of antimicrobial resistance only
- Many studies like Dhanya A et al. (6) also used Vitek-2 to confirm the diagnosis and antimicrobial resistance along with phenotypic methods, which can further increase the diagnostic and detection accuracy.
- Another limitation was that the sample size of the study is quite small and the findings cannot be generalized for a larger study population. The main purpose of this study was to find a general idea of the resistance pattern of the organism in our area and it may also pave the way for further elaborate studies involving a bigger study population and multiple centers for an integrated approach involving the analysis of beta-lactamase production and other mechanisms of multidrug resistance, especially in hospital settings.
Conclusion
The findings of the study indicate a change in susceptibility trends of emerging pathogens like Citrobacter spp. exhibiting resistance to routinely prescribed antimicrobials. Increasing antimicrobial resistance amongst such nosocomial pathogens is posing a serious challenge in the management of such infectionsstressing the need for an appropriate action plan involving antimicrobial stewardship, and strict implementation of infection control practices.
Appropriate Surveillance strategy for regular continuous monitoring of antimicrobial susceptibility patterns with the formulation of antibiotic policy at the institutional or regional level is an important prerequisite to rationalize antimicrobial treatment protocols to curb the menace of rapidly emerging drug resistance amongst such pathogens.
Acknowledgements
Nil
Ethical statement
This study was conducted in accordance with the ethical guidelines approved by the Institutional Ethics Committee (IEC) with the code 1346/IECBMHR/GMC/2024.
Author Contributions
All the authors have contributed significantly to the conception; acquisition, analysis, interpretation of data; drafting and reviewing of the manuscript. All the authors gave approval to the final version of the manuscript and agreed to be accountable for all aspects of the study.
Conflict of interest
There are no conflicts of interest.
Funding
Nil
Type of Study: orginal article |
Subject:
Microbiology
References
1. Winn WC, Koneman EW. Koneman's Color Atlas and Textbook of Diagnostic Microbiology. Lippincott Williams & Wilkins; 2006. [GOOGLE BOOKS]
2. Poonam AR, Bilolikar AK, Reddy SG. Prevalence and antimicrobial susceptibility pattern of Citrobacter species in various clinical samples in a tertiary care hospital. J Med Sci Res 2019;7(4):103-108. DOI: http://dx.doi.org/10.17727/JMSR.2019/7-18 [DOI:10.17727/JMSR.2019/7-18]
3. Kalpana S, Gajendra Varma. Antibiotic-Resistant Pattern of Citrobacter Species Isolated from Various Clinical Samples at VIMS Hospital, Ballari. J Microbiol Rel at Res 2019;5(2):71-74. [URL]
4. CLSI. Performance Standards for Antimicrobial Susceptibility Testing, M100 32nd Edition. Clinical and Laboratory Standards Institute, Wayne, PA; 2022. [URL]
5. Sadhna S, Hawaldar R. Prevalence and drug resistance pattern of Citrobacter spp. -A retrospective study. Indian J Microbiol Res 2019;6(2):142-5. [DOI:10.18231/j.ijmr.2019.030]
6. Nayar R, Shukla I, Sultan A. Epidemiology, Prevalence and Identification of Citrobacter Species in Clinical Specimens in a Tertiary Care Hospital in India. International Journal of Scientific and Research Publications 2014;4(4):1-6. [GOOGLE SCHOLAR]
7. Dhanya A, Bhat S. Clinicomicrobiological study of infections due to Citrobacter species. Journal of Evolution of Medical and Dental Sciences 2015;4:7327-31. DOI: 10.14260/jemds/2015/1063. [DOI:10.14260/jemds/2015/1063]
8. Dhason MT, Kamalasekaran LT, Chelliah A, Parthasarathy A, Suganthi, Manikeshi, et al. Prevalence of Citrobacter species in various clinical samples and their antibiotic susceptibility pattern study from a tertiary care center in South India. Int J Acad Med Pharm 2023;5(6):520-524. [GOOGLE SCHOLAR]
9. Ranjan KP, Ranjan Neelima. Citrobacter: An emerging healthcare-associated urinary pathogen. Urology Annals 2013;5(4):313-314. [DOI:10.4103/0974-7796.120297]
10. Metri, Basavaraj C, Jyothi P, Peerapur, Basavaraj V. Antibiotic resistance in Citrobacter spp. isolated from urinary tract infection. Urology Annals 2013;5(4):312-313. DOI: 10.4103/0974-7796.120295 [DOI:10.4103/0974-7796.120295] [PMID] []
11. Priyadarshini, Rani KL, Ramaswamy R. Isolation and antibiotic sensitivity pattern of Citrobacter species with ESBL and AmpC detection at Tertiary Care Hospital, Bangalore. J. Evolution Med. Dent Sci. 2016;5(30):1553-1556. DOI: 10.14260/jemds/2016/365 [DOI:10.14260/jemds/2016/365]
12. Sami H, Sultan A, Rizvi M, Fatima K, Ahmad S, Shukla I, Khan HM. Citrobacter as a uropathogen, its prevalence and antibiotics susceptibility pattern. CHRISMED Journal of Health and Research 2017;4(1):23-26. DOI: 10.4103/2348-3334.196037 [DOI:10.4103/2348-3334.196037]
13. Kumar S, Kumar S, Mittal S, Kumari P. Citrobacter spp. Isolated from Pus Samples in a Tertiary Care Hospital and its Antibiogram in Sonepat, Haryana, India. Int J Sci Stud 2020;7(12):14-17. [GOOGLE SCHOLAR]
14. Praharaj AK, Khajuria A, Kumar M, Grover N. Phenotypic detection and molecular characterization of beta-lactamase genes among Citrobacter species in a tertiary care hospital. Avicenna J Med 2016;6(1):17-27. DOI: 10.4103/2231-0770.173578 [DOI:10.4103/2231-0770.173578] [PMID] []
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







 gmail.com
gmail.com